Oct 14 2019
Liquid metal catalysts can potentially absorb carbon and remove contaminants. These catalysts could even be produced in the kitchen since they require very little energy.
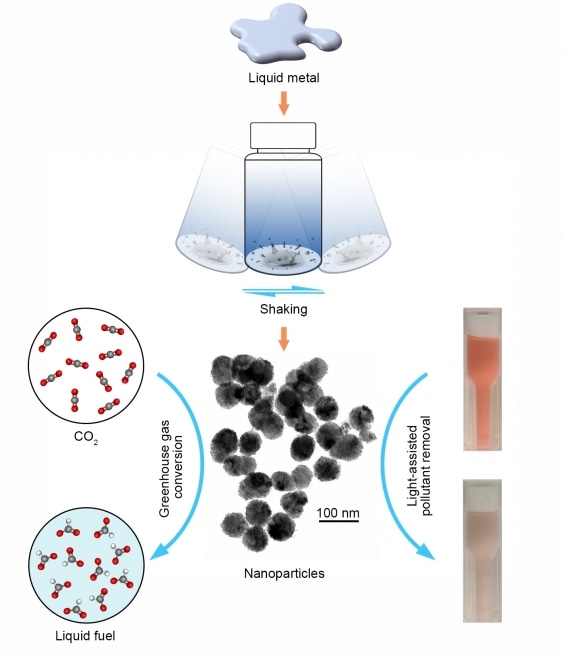 A diagram showing the process of producing liquid metal droplets which can then be used to capture carbon (left) or remove pollutants (right). (Image credit: UNSW)
A diagram showing the process of producing liquid metal droplets which can then be used to capture carbon (left) or remove pollutants (right). (Image credit: UNSW)
According to a study from UNSW Sydney, without the laboratory, substances that are capable of solving environmental issues by cleaning water, capturing CO2, and removing pollutants can be easily produced in a kitchen.
In a study reported in Nature Communications on October 14th, 2019, UNSW chemical engineers have tried to understand the mysterious realm of liquid metals and their role as catalysts to accelerate chemical reactions using less energy.
Anyone with a shaker and a cooktop at home in their kitchen can make catalysts that can be used for CO2 conversion, cleaning water and other pollutants. They can do this by using a combination of liquid metals like gallium, indium, bismuth and tin in alloys that can be melted under 300°C on a cooktop or in an oven.
Professor Kourosh Kalantar-Zadeh, School of Chemical Engineering, UNSW
Professor Kalantar-Zadeh and coworker Dr Jianbo Tang demonstrated that when an alloy of tin and bismuth was heated, the metal melted to a point considerably lower than when each metal was heated separately. Substances that show such behavior are called eutectic.
Eutectic alloys are the mixes of metals that produce the lowest melting point at a particular combination. For instance, if we combine bismuth at 57% and tin at 43% they melt at 139°C. But by themselves, both bismuth and tin have melting points above 200 °C.
Dr Jianbo Tang, School of Chemical Engineering, UNSW
Professor Kalantar-Zadeh remarked that the particular mix ratio of eutectic substances could produce the highest possible natural chaos at the nano-scale, which in turn reduces the melting point. The mechanism can also work vice versa. Eutectic metal substances that are already in the liquid form can solidify at a single temperature below the typical freezing point of each metal.
According to Professor Kalantar-Zadeh, “This maximum chaos helps, when we solidify the liquid metals, to naturally produce so many defects in the material that the ‘catalytic’ activity is significantly enhanced.”
How to Prepare a Liquid Metal Catalyst
Constituents: a eutectic alloy and water
- Heat a eutectic metal alloy in a saucepan on a high flame.
- Once the metal melts, cautiously pour it into a bottle containing water and fasten the cap.
- Shake both the liquid metal and water to form liquid metal droplets in water, just as shaking oil and vinegar to form oil droplets in the vinegar.
- Allow the droplets to solidify into a powder, which can now be used as a catalyst for electrochemically converting CO2.
Liquid Metals and the Environment
Liquid metal alloys could help in neutralizing or removing contaminants in the environment and absorbing the carbon in CO2 emissions. Gallium, tin, and bismuth in the liquid form are used as electrodes to convert CO2 into useful byproducts.
A further environmental application is that following the conversion of the liquid metals into oxides by heating, the substances could also be used to trap energy from light, which helps them to break down harmful chemicals in water.
The reason that liquid metals are an attractive alternative in solving environmental issues is that they could be generated in a cost-effective way using low-end technology and low energy.
Metals such as tin and bismuth are accessible to many people around the world. People should just consider how easily, cheaply and with so little need for advanced technology that they can be processed and transformed into useful materials such as catalysts. Additionally, playing with liquid metals is fun.
Professor Kourosh Kalantar-Zadeh, School of Chemical Engineering, UNSW
He added, “While the most famous liquid metal—mercury—is well known to be hazardous, a liquid metal like gallium is completely non-toxic, and meltable at or near room temperature, where we can use it to transform one material to another at very low input energies. Liquid metals could solve lots of problems that we as humans are grappling with these days.”
Professor Kalantar-Zadeh received the esteemed Australian Research Council (ARC) Laureate Fellowship, which awards funds for further research into liquid metals for another four years.