Around 80% of the human body is formed of water – an essential substance for all life. Increasing levels of pollution and land disturbance continue to cause huge quantities of potentially harmful elements to be mobilized into potable water sources, including groundwater, lakes and rivers.
Many of these elements elicit a toxicological response, requiring them to be strictly controlled throughout the world via international and national regulations.
One of the most frequently employed methods is the United States Environmental Protection Agency 200.8 (EPA 200.8).
All drinking waters are required to be tested prior to distribution to ensure they are fit for consumption. This results in high sample loads on water-testing laboratories.
In these settings, sample throughput is frequently hindered by extended uptake and washout times. Since these long run times often increase analysis costs, this also causes a substantial increase in a laboratory's routine testing overheads.
Where rapid throughput is essential, it is possible to significantly increase sample-to-sample analysis time through the implementation of selector valves and vacuum pumps, whereby a loop is filled with sample under vacuum, and the sample is introduced to the plasma at a consistent rate.
This approach enables high sample throughput because minimal time is required for the sample to enter the plasma. A system fully integrating this setup within its ICP-MS hardware and software has the potential to enhance laboratory throughput further.
This article explores the analysis of a series of tap and natural water samples in adherence with the EPA Method 200.8.
The study outlined here was performed using PerkinElmer's High Throughput System (HTS), integrated with the NexION® ICP-MS using Syngistix™ for ICP-MS software.
Experimental
Sample Preparation
Several isotopes and modes of analysis are recommended, depending on the elements in question. This study made use of HNO3 (67-70%, BDH Aristar® ULTRA, VWR, Mississauga, Ontario, Canada) to help keep the ions in solution.
A total of 200 µg/L Au was added to all samples and standards (including the carrier, internal standard and rinse solutions) in order to facilitate the washout of Hg. Calibration standards were prepared in 1% HNO3 and 200 ppb Au at specific levels (Table 1), while consumables used are listed in the table at the end of this article.
Table 1. Concentrations of calibration standards and internal standards used during analysis. Source: PerkinElmer, Inc.
| Analytes |
Standard
1 |
Standard
2 |
Standard
3 |
Standard
4 |
Standard
5 |
Ag, Al, As, Ba, Be, Cd, Co,
Cr, Cu, Fe*, Mn, Mo, Ni, Pb,
Sb, Se, Th, Tl, U, V, Zn |
1 |
5 |
10 |
50 |
100 |
| Hg |
0.05 |
0.1 |
0.5 |
1 |
2.5 |
| Ca*, K, Mg*, Na* |
50 |
100 |
1000 |
5000 |
10 000 |
| Au |
200 |
200 |
200 |
200 |
200 |
| Internal Standards |
71Ga, 72Ge, 103Rh, 159Tb |
*Not required by EPA 200.8, but included for informative value
Internal standards were added on-line via the seventh port (central channel) of the HTS system's valve. These were selected to accommodate the complete mass range of the analytes, covering the range of ionization efficiencies in the masses of interest.
Internal standard concentrations were varied to account for potential interferences, differences in ionization potential and isotopic abundance.
Table 2. Recommended isotopes and mode of analysis for different elements. Source: PerkinElmer, Inc.
| Analyte |
Recommended Isotope |
Correction Equation |
| Ag |
107 |
|
| Al |
27 |
|
| As |
75 |
-3.127 * [ArCl77 - (0.815 * Se82)] |
| Ba |
137 |
|
| Be |
9 |
|
| Ca* |
43 |
|
| Cd |
111 |
-1.073 * [MoO108 - (0.712 * Pd106)] |
| Co |
59 |
|
| Cr |
52 |
|
| Cu |
63 |
|
| Fe* |
54 |
- 0.0282 * Cr52 |
| Hg |
202 |
|
| K* |
39 |
|
| Mg* |
24 |
|
| Mn |
55 |
|
| Mo |
98 |
- 0.1096 * Ru101 |
| Na* |
23 |
|
| Ni |
60 |
|
| Pb# |
206 + 207 + 208 |
+ 1 * Pb206 + 1 * Pb207 |
| Sb |
121 |
|
| Se |
82 |
|
| Tl |
205 |
|
| Th |
232 |
|
| U |
238 |
|
| V |
51 |
-3.127 * [ClO53 - (0.013 * Cr52)] |
| Zn |
66 |
|
*Not required by EPA 200.8, but included for informative value
#Three isotopes should be monitored and summed
Inductively coupled plasma mass spectrometry (ICP-MS) was used to analyze tap and natural water samples in accordance with EPA 200.8. These were analyzed for 26 elements (Table 2).
EPA 200.8 prohibits the use of collision/reaction gases, prompting the study to employ mathematical correction equations to account for interferences (Table 2).
Calibration accuracy was validated via the implementation of an initial calibration verification standard (ICV) after the calibration. The method was then validated via the analysis of three certified reference materials (CRMs):
- Trace Metals in Drinking Water (High Purity Standards™, Charleston, South Carolina, USA)
- 1640a Natural Water and 1643f Water (NIST, Rockville, Maryland, USA)
The method's robustness was validated by implementing both low and high concentration spikes - 10 ppb and 50 ppb for every analyte defined in EPA 200.8, other than Hg, which utilized 0.5 ppb and 1 ppb for the low concentration and high concentration spikes, respectively.
These were conducted using a mixture of the analyte ions which covered the full range of analytes commonly present in drinking water.
Instrumentation
A NexION® ICP-MS (PerkinElmer Inc., Shelton, Connecticut, USA) was used to perform all analyses. This was fitted with a fully integrated HTS (PerkinElmer Inc.), with the components and parameters shown in Table 3.
Table 3. Component and parameters list for ICP-MS analysis. Source: PerkinElmer, Inc.
| Component/Parameter |
Type/Value |
| Nebulizer |
MEINHARD® plus Glass Type C |
| Spray Chamber |
Glass Cyclonic at room temperature |
| Sample Uptake Rate |
300 μL/min |
| RF Power |
1600 W |
| Injector |
2.0 mm I.D. quartz |
| Sweeps |
20 |
| Dwell Time |
15-100 ms (analyte dependent) |
| Replicates |
3 |
It can be difficult to analyze easily ionizable, high isotopic abundance analytes such as Na (100% isotopic abundance) and K (93% isotopic abundance) in drinking water samples.
This challenge is a result of potentially high concentrations, which may trigger a decrease in ICP-MS systems' detector lives when measuring the ion beam signal at full strength.
In these circumstances, analysts are advised to use the NexION ICP-MS's Extended Dynamic Range functionality (EDR).
The instrument uses a quadrupole ion guide as its universal cell, which can also serve as a collision/reaction cell for other applications. This means that per isotope, active adjustment of the transmission of ions is possible through the quadrupole cell.
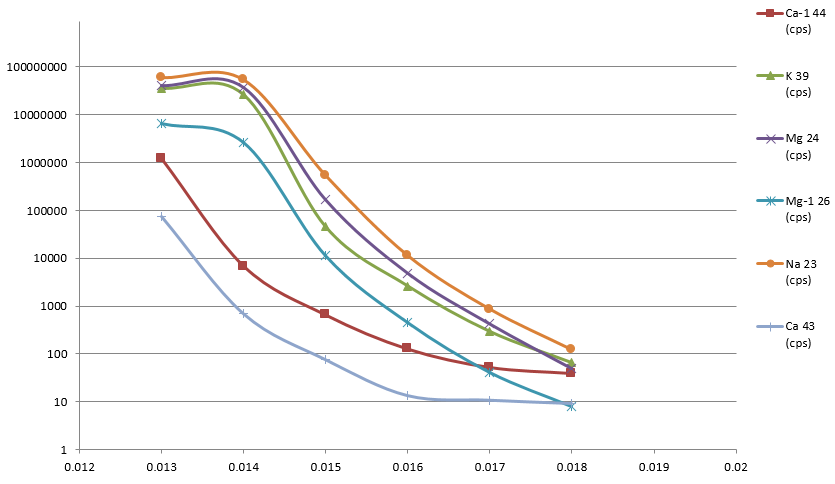
Figure 1. Effect of EDR on signal of analytes which are easily ionizable. Image Credit: PerkinElmer, Inc.
This approach allows attenuation of any analyte signals present in high abundance (Figure 1) prior to detection, therefore, extending the measurement's dynamic range by up to 12 orders of magnitude while also extending detector lifetime.
Ion transmission is maximized when analyzing low concentration analytes, facilitating the easy detection of low concentration analytes. This can all be performed in a single analytical method and run, saving analysts considerable time and effort.
Results and Discussion
A NexION ICP-MS was used to analyze every sample for 27 different analytes. The fully integrated HTS system enabled a 3-5x reduction in sample-to-sample times versus normal analysis, saving on both time and costs.
Ten repeat injections of a sample were run to evaluate short-term precision. These spanned the low, mid and high mass ranges.
Precision was found to be <1.5% for all analytes other than Hg, which was slightly over 2% - confirming that the degree of variance between replicate measurements is insignificant and not affected by the HTS system.
Figure 2. Sample introduction into the plasma in less than 10 seconds. Image Credit: PerkinElmer, Inc.
It was possible to observe water samples in the plasma within 9 seconds of sample probe immersion (Figure 2), illustrating that the sample is present in the plasma and remains stable within a very short time versus standard uptake methods.
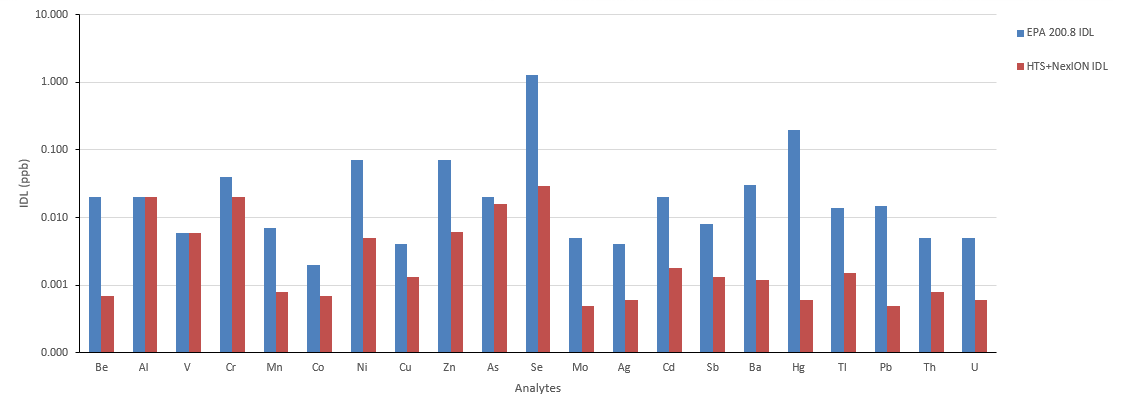
Figure 3. Detection limits using HTS system compared to those reported in the EPA 200.8. Image Credit: PerkinElmer, Inc.
Figure 3 displays instrument detection limits for the integrated HTS and ICP-MS system using 1% HNO3. These values are plotted against the EPA 200.8-specified values, with observed concentrations found to be considerably lower than those reported in the EPA document.
These findings confirmed that the HTS system's metal-free fluid path does not have any notable effect on the detection limits and that these are more than sufficient to accommodate the needs of this application.
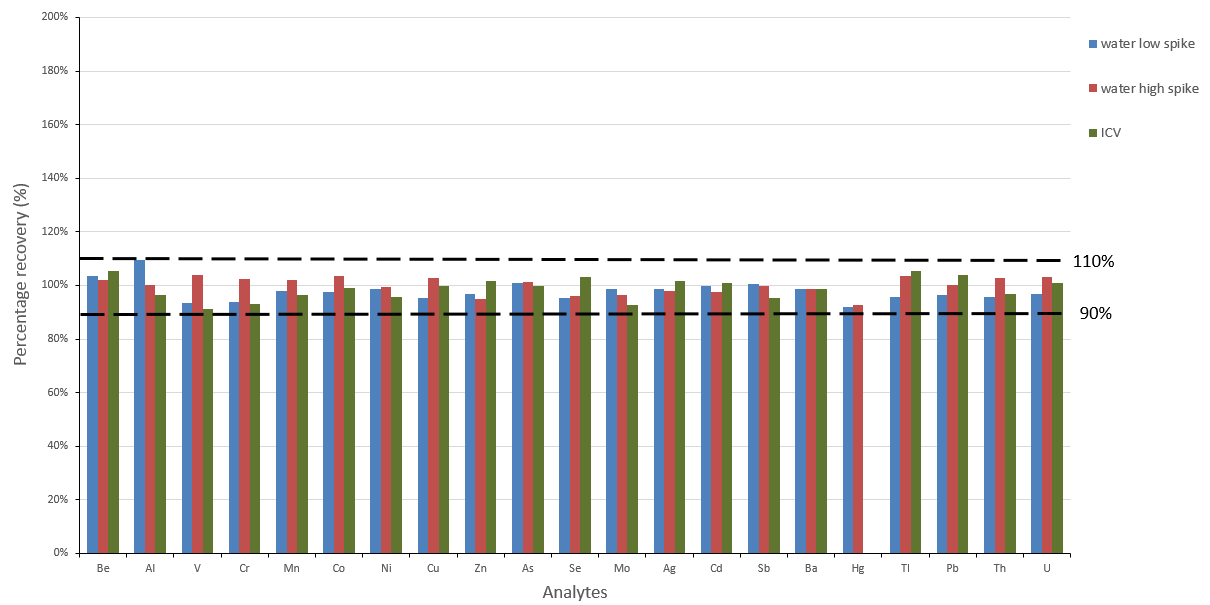
Figure 4. Initial calibration standard, low and high concentration spike recoveries. Image Credit: PerkinElmer, Inc.
Calibration was validated via an initial calibration verification standard (ICV), which was measured following the calibration standards and was determined to agree with the anticipated concentrations (Figure 4).
Additional quality controls were performed involving five replicate analyses of both a low- and high-spiked water sample. These checks confirmed the good recovery of all analyte spikes and further demonstrated the method's robustness and applicability over a considerable linear range (Figure 4).
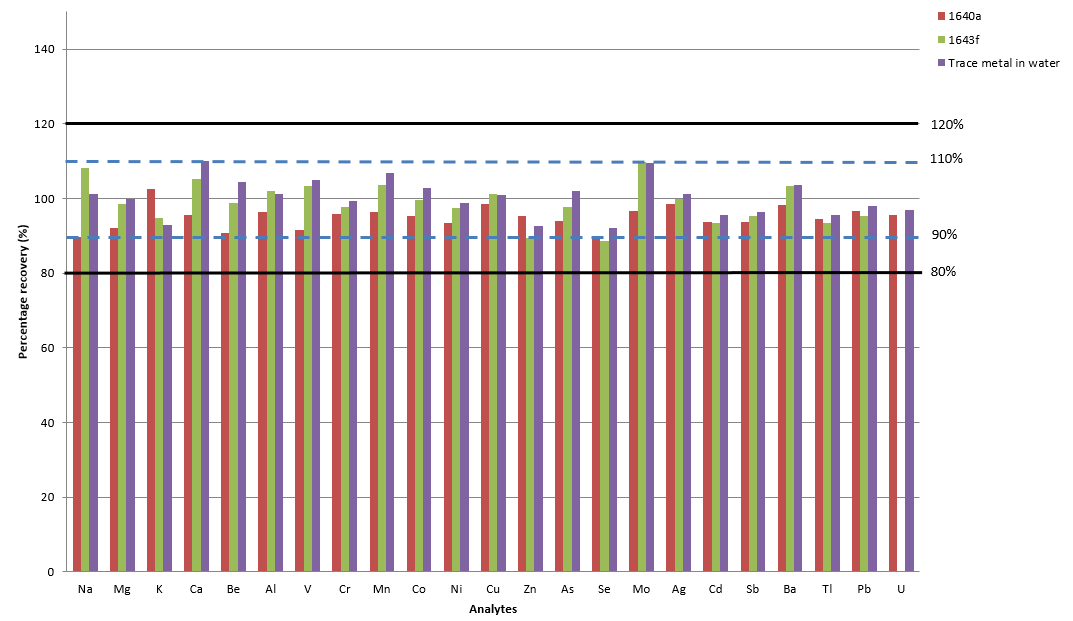
Figure 5. Certified reference material recoveries. Image Credit: PerkinElmer, Inc.
Figure 5 displays average recoveries of five repeat CRM material injections, highlighting that most of the analyte recoveries fell within 10% of the CRM's certified value for NIST 1643e, NIST 1643f and Trace Metals in Water.
The only exception to this was the value for Se, which was found to be 89% and 88% for 1640a and 1643f, respectively. These findings further confirmed the method's analytical accuracy.
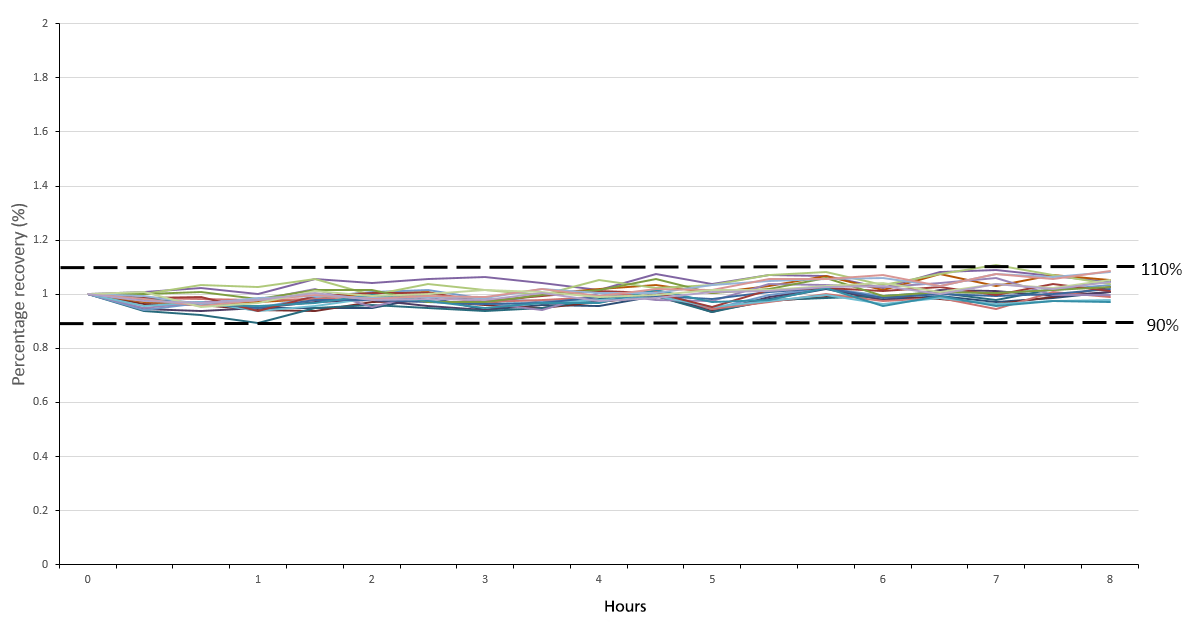
Figure 6. Percentage recovery of the continuing calibration verification standard over a period of 8 hours. Image Credit: PerkinElmer, Inc.
The method's stability was evaluated by running a continuing calibration standard (CCV) every 10 samples and internal standards in every sample. This was done over the course of 8 hours to represent a standard work shift (Figure 6).
The CCV demonstrated excellent stability (<10% RSD) over the whole analysis period. There was no need to recalibrate, highlighting the system's ability to provide consistent, reliable and accurate results over extended run times – a key consideration for many high-throughput laboratories.
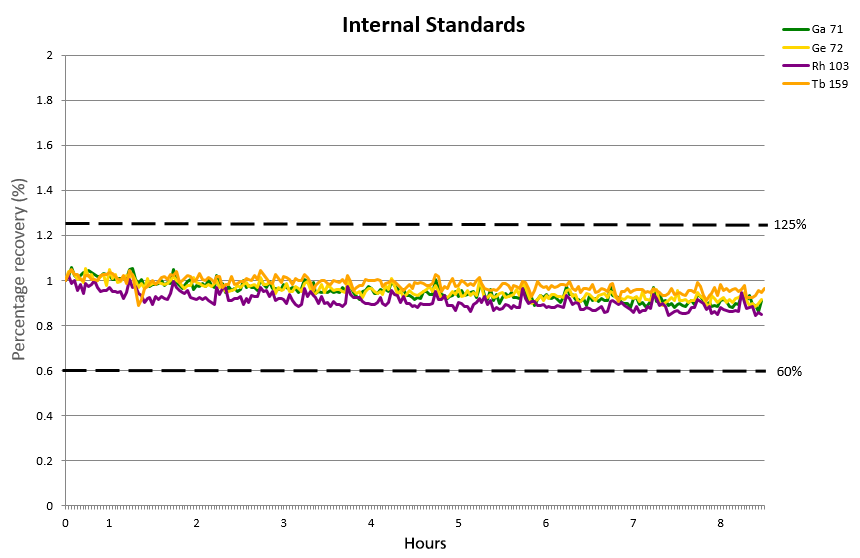
Figure 7. Internal standard over a period of 8 hours. Image Credit: PerkinElmer, Inc.
The internal standard did not deviate more than ±15% over the 8-hour period (Figure 7) and was found to be confidently within the limits of 60-125% recovery permitted by EPA 200.8.
This further confirms the HTS system's suitability and applicability for the routine analysis of trace metals and metalloids in both tap and natural water samples.
Conclusion
This article explored the use of a high throughput method to evaluate 27 different analytes in drinking water, in line with EPA 200.8.
The HTS system - fully integrated with the NexION ICP-MS – enabled a dramatic reduction in sample analysis time by 3-5x without compromising the analysis' accuracy, repeatability or detection limits.
The system was found to offer excellent precision, with long-term stability falling within 10% for all analytes – a result determined via the use of a CCV over an 8-hour analytical run.
These convincing results serve to highlight the suitability of this system for high-throughput laboratories, illustrating its potential to reduce analysis cost and time significantly.
Accessories and Consumables Used
Table 4. Source: PerkinElmer, Inc.
| Component |
Part Number |
| PerkinElmer HTS System |
N8150141 |
| Instrument Calibration Standard 2 |
N9301721 |
| Environmental Standard Mix B, Th, U |
N9307807 |
| Environmental Standard Mix Ca, K, Mg, Na |
N9307805 |
| Mercury Standard |
N9300253 |
| Ga Standard |
N9303772 |
| Ge Standard |
N9303739 |
| Rh Standard |
N9303749 |
| Tb Standard |
N9303778 |
| Au Standard |
N9303759 |
| Autosampler Tubes |
N0776118 (15 mL)
N0776116 (50 mL) |
Acknowledgments
Produced from materials originally authored by Ram Lamsal from Queen's University, Department of Chemistry and Eve Kroukamp from PerkinElmer Inc.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer, Inc.
For more information on this source, please visit PerkinElmer, Inc.