The quality of potable water can be influenced by various factors, such as geology, topography, pollution, and leaching from water transport pipelines. Thus, a strong understanding of water quality is of the utmost importance when it comes to both industrial applications and consumption.

Image Credit: ShutterStock/Cozine
The main elements to monitor include aluminum (Al), iron (Fe), manganese (Mn), copper (Cu), and zinc (Zn), as well as minerals: magnesium (Mg), calcium (Ca), sodium (Na), and potassium (K). The importance of these elements is highlighted below in Table 1.
Table 1. Elements Important in Water Treatment. Source: PerkinElmer
| Element |
Importance |
| Al |
Lower levels needed for renal analysis - < 0.1 mg/L |
| Cu |
Corrosion of pipes and fittings; > 2 mg/L can cause negative health effects |
| Fe |
High concentrations can stain |
| Mn |
Causes staining and affects taste |
| Zn |
Corrosion from galvanized pipes and fittings as well as brass |
| Minerals (Ca, Mg, K, Na) |
Indication of hardness and scaling potential; affects taste |
Conventional methods utilized in water laboratories for elemental analysis include atomic ion chromatography (IC), absorption spectrometry (AAS), and flow injection analysis (FIA). These more traditional methods tend to be time-consuming and rather slow, often necessitating three separate pieces of equipment to achieve the end result.
An easy method for elemental analysis in potable waters would be ICP-OES, a multielement method with a wide dynamic range.
As a substitute for elements traditionally assessed via AAS, IC, and FIA, PerkinElmer's Avio° 220 Max hybrid simultaneous ICP-OES delivers exceptional performance with reduced operational expenses in contrast to a fully simultaneous ICP-OES system. This configuration is particularly well-suited for laboratories handling moderate sample workloads.
This article will focus on the analysis of potable waters using the Avio 220 Max hybrid simultaneous ICP-OES, measuring elements that are typically analyzed by AAS, IC, and FIA at required levels.
Experimental
Samples
Accuracy was determined by examining a certified reference material (Trace Metals in Drinking Water B, High Purity Standards, Charleston, South Carolina, USA), whose concentrations are displayed in Table 2. Tap water samples were gathered and acidified to 1% nitric acid (v/v) before analysis.
An internal standard was added to all blanks, standards, and samples. All measurements were made against external calibration curves with the help of the calibration standards in Table 3 prepared in 1% nitric acid (v/v) and a linear through zero regression.
Table 2. Analyte Concentrations in TMDW-B Certified Reference Material. Source: PerkinElmer
| Element |
Concentration (mg/L) |
| Al |
0.125 |
| Ca |
31 |
| Cu |
0.020 |
| Fe |
0.090 |
| K |
2.5 |
| Mg |
8 |
| Mn |
0.040 |
| Na |
22 |
| Zn |
0.075 |
Table 3. Calibration Standards. Source: PerkinElmer
| Element |
Standard 1 (mg/L) |
Standard 2 (mg/L) |
Standard 3 (mg/L) |
Standard 4 (mg/L) |
| Mn |
0.01 |
0.02 |
0.04 |
0.1 |
| Al, Cu, Fe, Zn |
0.1 |
0.2 |
0.4 |
1 |
| Ca, K, Mg, Na |
5 |
10 |
20 |
40 |
Instrumental Conditions
Analyses were executed on an Avio 220 Max hybrid simultaneous ICP-OES utilizing the conditions in Table 4 and the wavelengths and plasma views in Table 5.
One major benefit of the Avio 220 Max is its outstanding sensitivity as a result of its unique optical design and detector.2,3 The improved sensitivity enables shorter integration times to be used, thereby decreasing total analysis time.
By using the Auto Integration capability, the instrument automatically determines the appropriate integration time per analyte, an ideal scenario when measuring elements with both low and high concentrations in the same sample.
In addition, Dual View also enables analyses of both trace and major elements by providing the ability to view any analyte in either axial and/or radial view within the same method, without making compromises on wavelength selection.
Table 4. Avio 220 Max ICP-OES Conditions/Parameters. Source: PerkinElmer
| Component / Parameter |
Description / Value |
| Nebulizer |
MEINHARD® K1, LDV |
| Spray Chamber |
Glass cyclonic |
| Injector |
2.0 mm i.d. alumina |
| RF Power |
1500 W |
| Nebulizer Flow |
0.70 L/minute |
| Auxiliary Flow |
0.2 L/minute |
| Plasma Flow |
8 L/minute |
| Integration |
Auto, 0.5 – 2 seconds |
| Replicates |
2 |
| Sample Uptake Rate |
1 mL/minute |
| Sample Uptake Tubing |
Black/black (0.76 mm id), PVC |
| Drain Tubing |
Gray/gray (1.30 mm id), Santoprene |
Table 5. Elements, Wavelengths, and Plasma Views. Source: PerkinElmer
| Element |
Wavelength (nm) |
Plasma View |
| Al |
308.215 |
Axial |
| Ca |
317.935 |
Radial |
| Cu |
327.393 |
Axial |
| Fe |
238.206 |
Axial |
| Mg |
285.214 |
Radial |
| Mn |
257.610 |
Axial |
| K |
766.490 |
Radial |
| Na |
589.592 |
Radial |
| Zn |
206.200 |
Axial |
| Y (internal standard) |
371.029 |
Axial and Radial |
Results and Discussion
Accuracy was determined through the analysis of a certified reference material, which was measured three times. As shown in Figure 1, all recoveries were constantly within 10% of their certified value.
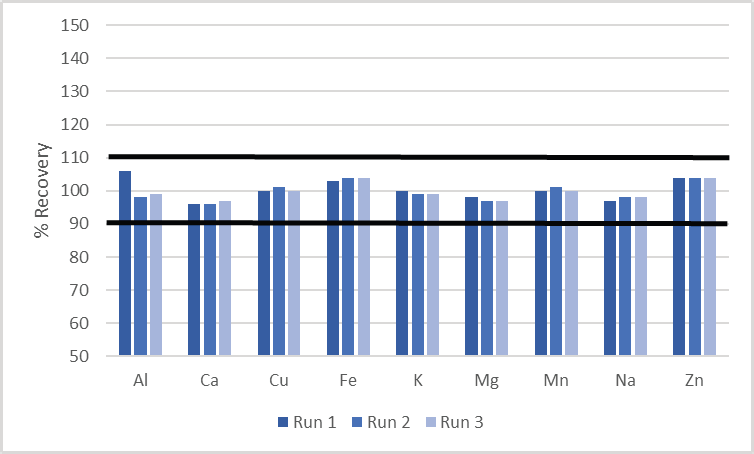
Figure 1. Recoveries in Trace Metals in Drinking Water B, over three analyses. Image Credit: PerkinElmer
With the accuracy now established, several tap water samples were also quantified (Figure 2), with variations being noted between the samples. As predicted, the mineral content was greater compared to the other elements and comparatively constant, with changes of below one order of magnitude between the samples.
Aluminum (Al) was absent in all samples, and manganese (Mn) was only quantifiable in four samples at levels below 0.010 mg/L. Copper (Cu) was seen in all samples with changes of two orders of magnitude, most likely indicating that there was leaching from the Cu pipe.
Although iron (Fe) was detected across all samples at minor concentrations, the measured zinc (Zn) levels exhibited significant variability, ranging from non-detectable in two samples to exceeding 1 mg/L in others. Such outcomes illustrate that the methodology could help distinguish water samples.
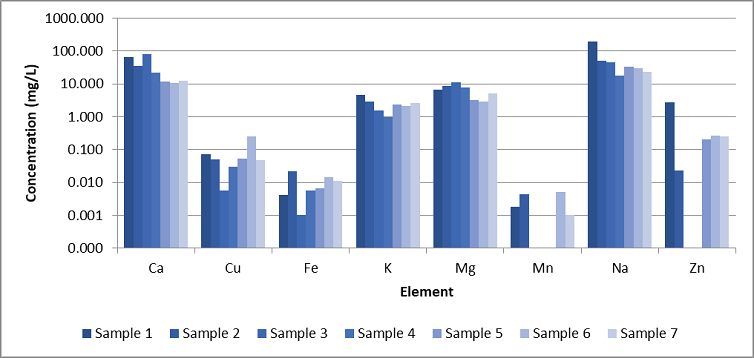
Figure 2. Results from the analysis of seven different potable water samples. Aluminum (Al) was not detected in any of the samples. Image Credit: PerkinElmer
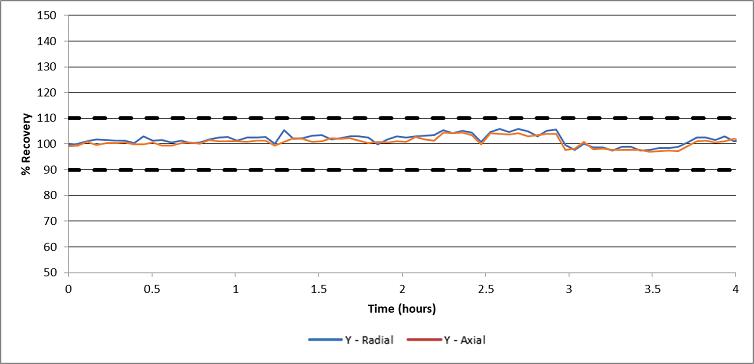
Figure 3. Internal standard recoveries during a 4-hour drinking water analysis. Image Credit: PerkinElmer
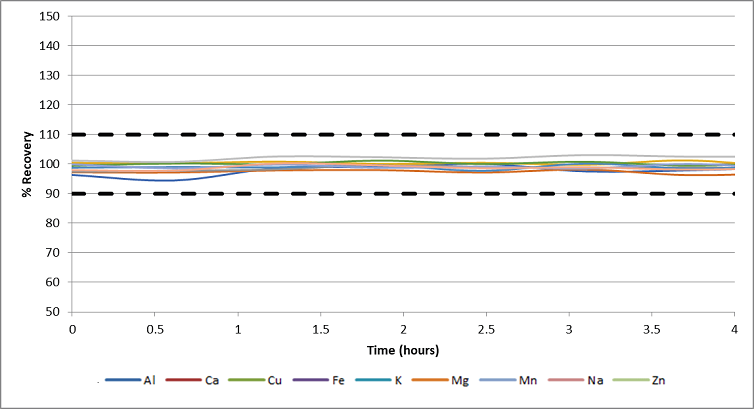
Figure 4. Recovery of check standard during a 4-hour drinking water analysis. Image Credit: PerkinElmer
Even though the elements of interest are normally present at high concentrations (with the exceptions of Mn and Al), method detection limits (MDLs) were identified with the Detection Limit functionality in Syngistix™ for ICP software as three times the standard deviation of 10 replicates of a low-level standard whose concentration is displayed in Table 6.
The detection limits for all elements are 1 to 3 orders of magnitude below the average values quantified in the sample (Figure 5), meaning that results in typical potable waters can be obtained with high confidence.
Table 6. Concentrations in Detection Limit Standard. Source: PerkinElmer
| Element |
Concentration (mg/L) |
| Mn |
0.001 |
| Al, Cu, Fe, Zn |
0.01 |
| Ca, K, Mg, Na |
0.5 |
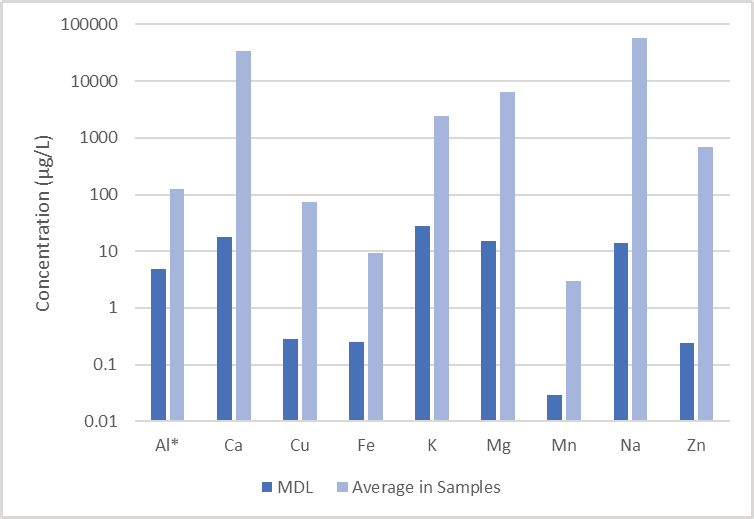
Figure 5. Detection limits measured in a low-level standard, compared to the average values found in the analyzed samples. (* since Al was not detected, its concentration in the CRM is plotted.) Image Credit: PerkinElmer
Conclusion
This article has illustrated how PerkinElmer’s Avio 220 Max hybrid concurrent ICP-OES can be used to successfully quantify nine elements in potable water.
The main benefit of the Avio 220 Max is enhanced productivity in comparison to older methods like AAS while offering lower operating costs compared to completely simultaneous ICP instruments.
Other advantages of the Avio 220 Max for water treatment plants include the potential to precisely quantify lower concentrations as well as add new elements without adding considerable analytical time, should the need arise.
References
- “Australian Drinking Water Guidelines”, Version 3.6, pp. 170-190.
- “The Avio 220 Max ICP-OES: A Unique Double-Monochromator Optical System”, Technical Note, PerkinElmer, 2020.
- “Avio 220 Max ICP-OES Custom-Designed Solid-State Detector with Hybrid Simultaneous Analysis”, Technical Note, PerkinElmer, 2020.3.
Consumables Used
Table 7. Source: PerkinElmer
| Component |
Part Number |
| Sample Uptake Tubing: Black/Black (0.76 mm i.d.), PVC, Flared |
N0777043 |
| Drain Tubing: Gray/Gray (1.30 mm i.d.), Santoprene |
N0777444 |
| Autosampler Tubes (quantity = 500) |
B0193233 (15 mL)
B0193234 (50 mL) |
| Single-Element Standard: Aluminum, 1000 ppm |
N9300184 (125 mL)
N9300100 (500 mL) |
| Single-Element Standard: Copper, 1000 ppm |
N9300183 (125 mL)
N9300114 (500 mL) |
| Single-Element Standard: Iron, 1000 ppm |
N9303771 (125 mL)
N9300126 (500 mL) |
| Single-Element Standard: Manganese, 1000 ppm |
N9303783 (125 mL)
N9300132 (500 mL) |
| Single-Element Standard: Zinc, 1000 ppm |
N9300178 (125 mL)
N9300168 (500 mL) |
| Instrument Calibration Standard 1: Ca, K, Mg, Na, 5000 ppm each |
N9300218 (125 mL) |