Scientists have been trying to achieve artificial photosynthesis for the generation of solar hydrogen. The process of photosynthesis uses water and carbon dioxide to convert solar energy into storable fuel. Researchers have been endeavoring to imitate and implement this process for photo-electrochemical cells (PEC) and similar energy device applications.
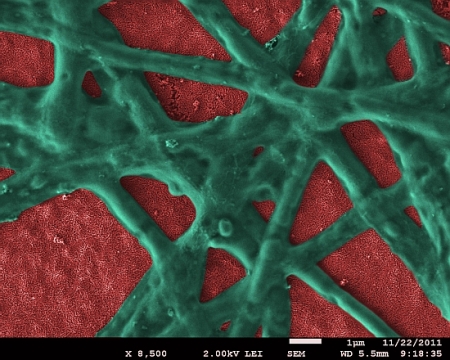 Hematite nanoparticle film (red) with functional phycocyanin network (green) attached. (Image by Dr. E. Vitol, Argonne National Laboratory)
Hematite nanoparticle film (red) with functional phycocyanin network (green) attached. (Image by Dr. E. Vitol, Argonne National Laboratory)
For generating hydrogen, PECs use sunlight for splitting water electrochemically. This process is shorter than those using photovoltaic cells for performing electrolysis of water. PEC electrodes are usually made from semiconducting materials, and some of these materials possess photocatalytic properties.
Empa researchers at the Laboratory for High Performance Ceramics (LHPC) have been conducting research on the nanoparticles of these semiconducting materials for neutralizing organic pollutants found in water and air. The researchers partnered with Argonne National Laboratory and the University of Basel and developed a nano-bio PEC electrode. This electrode has double the efficiency of iron oxide in splitting water. The nano-bio PEC electrode consists of iron oxide that is conjugated with a protein derived from cyanobacteria or blue-green algae.
Iron oxide,especially hematite uses sunlight more efficiently than titanium dioxide (TiO2) and other photocatalysts, which have the capability to use only the UV part of solar radiation. As hematite material is susceptible to visible wavelengths, they are a better option for PECs.
Phycocyanin is a protein from blue-green algae. It is an important light-harvesting component of the bacteria. Debajeet K. Bora, a researcher who performed his PhD thesis at Empa, designed the new electrode using proteins and ceramics. Surface functionalization of hematites with proteins is a new concept in the research of PECs. Researcher Bora cross-coupled phycocyanin to the hematite nanoparticles covalently. This made the compounded hematite to absorb more photons. When compared to an iron oxide electrode, the photocurrent induced was double.
As a surprising benefit, even under strong illumination in an alkaline environment, the light harvesting protein complex also is not destroyed when it comes in contact with a photocatalyst. In this situation, the organic molecules survive the harsh photocatalytic conditions and also double the photocurrent.