Jan 23 2014
Researchers at The University of Texas at Austin's Cockrell School of Engineering have developed a new source of renewable energy, a biofuel, from genetically engineered yeast cells and ordinary table sugar. This yeast produces oils and fats, known as lipids, that can be used in place of petroleum-derived products.
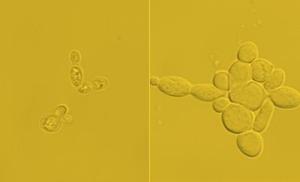 This image shows: (L) Starting cells with around 15% lipid content. (R) Engineered cells with nearly 90% lipid content. A team at The University of Texas at Austin's Cockrell School of Engineering has created a platform for converting yeast cells into lipids, which can then become biofuel and other petroleum-based products. (credit: Cockrell School of Engineering, The University of Texas at Austin)
This image shows: (L) Starting cells with around 15% lipid content. (R) Engineered cells with nearly 90% lipid content. A team at The University of Texas at Austin's Cockrell School of Engineering has created a platform for converting yeast cells into lipids, which can then become biofuel and other petroleum-based products. (credit: Cockrell School of Engineering, The University of Texas at Austin)
Assistant professor Hal Alper, in the Cockrell School's McKetta Department of Chemical Engineering, along with his team of students, created the new cell-based platform. Given that the yeast cells grow on sugars, Alper calls the biofuel produced by this process "a renewable version of sweet crude."
The researchers' platform produces the highest concentration of oils and fats reported through fermentation, the process of culturing cells to convert sugar into products such as alcohol, gases or acids. This work was published in Nature Communications on Jan. 20.
The UT Austin research team was able to rewire yeast cells to enable up to 90 percent of the cell mass to become lipids, which can then be used to produce biodiesel.
"To put this in perspective, this lipid value is approaching the concentration seen in many industrial biochemical processes," Alper said. "You can take the lipids formed and theoretically use it to power a car."
Since fatty materials are building blocks for many household products, this process could be used to produce a variety of items made with petroleum or oils — from nylon to nutrition supplements to fuels. Biofuels and chemicals produced from living organisms represent a promising portion of the renewable energy market. Overall, the global biofuels market is expected to double during the next several years, going from $82.7 billion in 2011 to $185.3 billion in 2021.
"We took a starting yeast strain of Yarrowia lipolytica, and we've been able to convert it into a factory for oil directly from sugar," Alper said. "This work opens up a new platform for a renewable energy and chemical source."
The biofuel the researchers formulated is similar in composition to biodiesel made from soybean oil. The advantages of using the yeast cells to produce commercial-grade biodiesel are that yeast cells can be grown anywhere, do not compete with land resources and are easier to genetically alter than other sources of biofuel.
"By genetically rewiring Yarrowia lipolytica, Dr. Alper and his research group have created a near-commercial biocatalyst that produces high levels of bio-oils during carbohydrate fermentation," said Lonnie O. Ingram, director of the Florida Center for Renewable Chemicals and Fuels at the University of Florida. "This is a remarkable demonstration of the power of metabolic engineering."
So far, high-level production of biofuels and renewable oils has been an elusive goal, but the researchers believe that industry-scale production is possible with their platform.
In a large-scale engineering effort spanning over four years, the researchers genetically modified Yarrowia lipolytica by both removing and overexpressing specific genes that influence lipid production. In addition, the team identified optimum culturing conditions that differ from standard conditions. Traditional methods rely on nitrogen starvation to trick yeast cells into storing fat and materials. Alper's research provides a mechanism for growing lipids without nitrogen starvation. The research has resulted in a technology for which UT Austin has applied for a patent.
"Our cells do not require that starvation," Alper said. "That makes it extremely attractive from an industry production standpoint."
The team increased lipid levels by nearly 60-fold from the starting point.
At 90 percent lipid levels, the platform produces the highest levels of lipid content created so far using a genetically engineered yeast cell. To compare, other yeast-based platforms yield lipid content in the 50 to 80 percent range. However, these alternative platforms do not always produce lipids directly from sugar as the UT Austin technology does.
Alper and his team are continuing to find ways to further enhance the lipid production levels and develop new products using this engineered yeast.