May 22 2017
The demand for development of renewable energy sources is growing as there is widespread concern regarding the energy crisis and the seriousness of environmental contamination. These renewable energy sources must be feasible alternatives to diminishing fossil fuels.
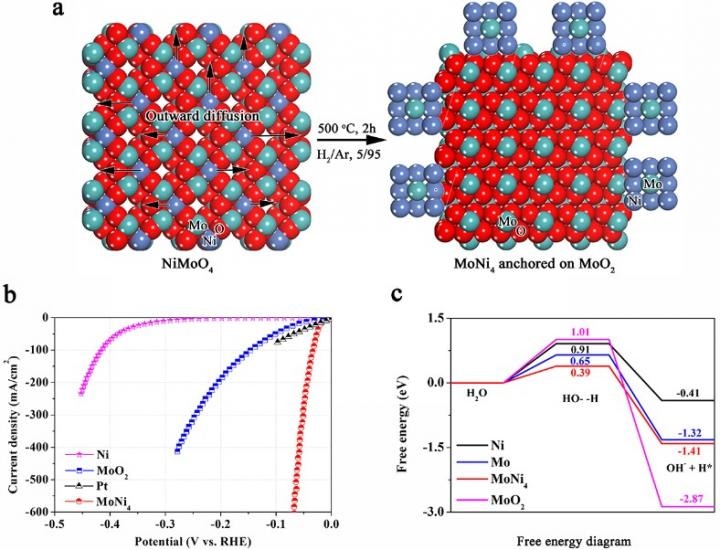 This figure shows: a) Synthetic scheme of MoNi4 electrocatalyst supported by the MoO2 cuboids on nickel foam; b) polarization curves of the MoNi4 electrocatalyst supported by the MoO2 cuboids, pure Ni nanosheets and MoO2 cuboids on the nickel foam; c) calculated adsorption free energy diagram for the Tafel step. (CREDIT - Xinliang Feng/cfaed)
This figure shows: a) Synthetic scheme of MoNi4 electrocatalyst supported by the MoO2 cuboids on nickel foam; b) polarization curves of the MoNi4 electrocatalyst supported by the MoO2 cuboids, pure Ni nanosheets and MoO2 cuboids on the nickel foam; c) calculated adsorption free energy diagram for the Tafel step. (CREDIT - Xinliang Feng/cfaed)
Due to its high energy density and environmentally friendly features, molecular hydrogen is an appealing and promising energy carrier to match future global energy demands. In a number of the methods for hydrogen production, the electrocatalytic hydrogen evolution reaction (HER) from water splitting is the most effective and economical route for the future hydrogen economy. To speed up the sluggish HER kinetics, mainly in alkaline electrolytes, very active and durable electrocatalysts are important to lower the kinetic HER overpotential. As a standard HER electrocatalyst with a zero HER overpotential, the precious metal platinum (Pt) has a dominant role to play in current H2-production technologies, such as water-alkali electrolysers. Regrettably, the high cost and scarcity of Pt seriously hinder its large-scale applications in electrocatalytic HERs.
Prof. Xinliang Feng's team from the Technische Universität Dresden (Germany)/ Center for Advancing Electronics Dresden (cfaed), in partnership with the University Lyon, ENS de Lyon, Centre national de la recherche scientifique (CNRS, France), the Tohoku University (Japan) and the Fraunhofer Institute for Ceramic Technologies and Systems (IKTS) (Germany), have developed an economical MoNi4 electrocatalyst anchored on MoO2 cuboids, which are vertically aligned on nickel foam (MoNi4/MoO2@Ni). MoNi4 nanoparticles are built in situ on the MoO2 cuboids by manipulating the outward diffusion of Ni atoms. The resultant MoNi4/MoO2@Ni displays a high HER activity that is highly comparable to that of the Pt catalyst and offers advanced HER activity among all reported Pt-free electrocatalysts. Experimental investigations reveal that the MoNi4 electrocatalyst acts as the very active center and displays quick Tafel step-determined HER kinetics. Moreover, density functional theory (DFT) calculations establish that the kinetic energy barrier of the Volmer step for the MoNi4 electrocatalyst is highly decreased. The large-scale preparation and superior catalytic stability offer MoNi4/MoO2@Ni with a potential utilization in water-alkali electrolysers for hydrogen production. Thus, the exploration and appreciation of the MoNi4 electrocatalyst offer a potential alternative to Pt catalysts for emerging applications in energy generation.