Nov 16 2017
Modern solar cells, which make use of energy from light in order to produce holes and electrons that are then transported out of semiconducting materials and into external circuits to be used by humans, have been present in one form or another for more than 60 years.
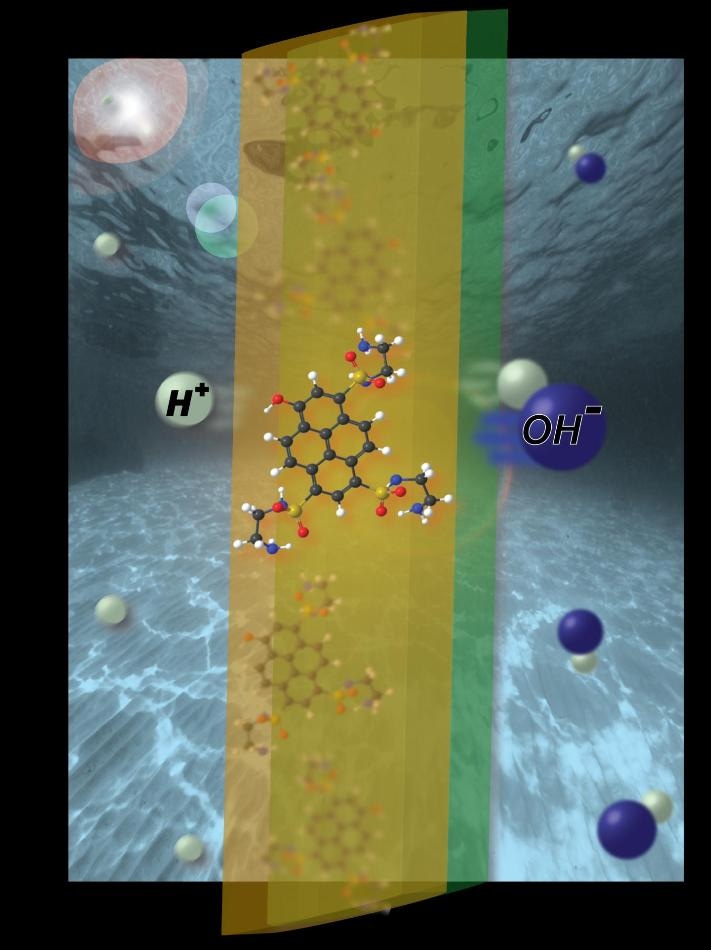
Minimal attention has been paid, however, to the promise of employing light to drive another electricity-generating process -- the transport of oppositely charged protons and hydroxides attained by dissociating water molecules. Researchers in America report such a design, which has potential application in producing electricity to turn brackish water drinkable, on November 15 in the journal Joule.
 This is an artist's rendition of bipolar-membrane design for ionic electricity generation. CREDIT William White
This is an artist's rendition of bipolar-membrane design for ionic electricity generation. CREDIT William White
The researchers, headed by senior author Shane Ardo, an Assistant Professor of Chemistry, Chemical Engineering, and Materials Science at the University of California, Irvine, writes that they have created an "ionic analog to the electronic pn-junction solar cell," capable of harnessing light in order to exploit the semiconductor-like behavior of water and produce ionic electricity. They expect to use such a mechanism for manufacturing a device capable of directly desalinating saltwater upon exposure to sunlight.
There had been other experiments dating back to the 1980s that photoexcited materials so as to pass an ionic current through them, and theoretical studies said that those currents should be able to reach the same levels as their electronic analogs, but none of them worked all that well.
William White, first author, graduate student in Ardo's research group.
In this case, the researchers gained more success by allowing water to pass via two ion-exchange membranes, one that mostly transported positively charged ions (cations) like protons and one that mostly transported negatively charged ions (anions) like hydroxides, operating as a pair of chemical gates in order to get charge separation. Shining a laser on the system provoked light-sensitive organic dye molecules bound to the membrane in order to liberate protons, which then transported to the more acidic side of the membrane and generated a measurable ionic current and voltages of more than 100 mV in some instances (60 mV on average).
In spite of crossing the 100 mV photovoltage threshold at times, the level of electric current that the double-membrane system can attain remains its chief limitation. The photovoltage will have to be magnified by more than another factor of two in order to reach the ~200 mV mark necessary to desalinate seawater, a target that the researchers are optimistic about hitting.
"It all comes down to the fundamental physics of how long the charge-carriers persist before recombining to form water," Ardo says. "Knowing the properties of water, we are able to more intelligently design one of these bipolar-membrane interfaces so that we can maximize the voltage and the current."
Going forward, desalination is just one possible application of the synthetic light-driven proton pump created by the researchers. It could also have likelihood for interfacing with electronic devices, or also for powering signaling in brain-machine interfaces and other "cyborg cells" that integrate living tissue and artificial circuity, a role that cannot be filled by standard solar cells, which are considered to be unstable in biological systems.
We have had a lot of ideas about what this technology could be used for; it's just a question of learning enough to cross between fields and make the device work for those intended applications, I think this is just another example of what you can do when you have scientists who are trained across many disciplines and think outside the box.
Shane Ardo, an Assistant Professor of Chemistry, Chemical Engineering, and Materials Science at the University of California