Mar 7 2018
A new, dual-atom catalyst has been synthesized by scientists from the United States and China to function as a platform for artificial photosynthesis, holding the potential to provide solutions to more efficient harvesting and storage of solar energy.
The study results have been reported in the Proceedings of the National Academy of Sciences journal.
An iridium catalyst featuring only two active metal centers has been developed by the research team. Most notably, experimental results revealed that the catalyst has a well-defined structure with the ability to function as a productive platform for upcoming research works on solar fuel synthesis.
Our research concerns the technology for direct solar energy storage. Stable Iridium Dinuclear Heterogeneous Catalysts Supported on Metal-Oxide Substrate for Solar Water Oxidation. It addresses the critical challenge that solar energy is intermittent. It does so by directly harvesting solar energy and storing the energy in chemical bonds, similar to how photosynthesis is performed but with higher efficiencies and lower cost.
Dunwei Wang, Lead Author & Boston College Associate Professor of Chemistry
Scientists have spent a significant amount of time on single-atom catalysts (SACs) and seldom studied an "atomically dispersed catalyst" containing two atoms. In their report, the researchers have explained about the synthesis of an iridium dinuclear heterogeneous catalyst in a facile photochemical way. Their catalyst exhibits high activity toward water oxidation and excellent stability. The former characteristic is a vital process in both natural and artificial photosynthesis.
Researchers are exploring this aspect of catalysis experience specific challenges in the creation of heterogeneous catalysts, which are commonly used in large-scale industrial chemical transformations. A majority of the active heterogeneous catalysts often have their atomic structures poorly defined, posing challenges to investigate the in-depth mechanisms at the molecular level.
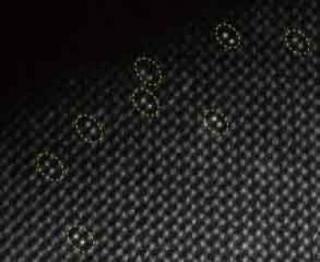 A structural characterization of a new iridium dinuclear heterogeneous catalyst reveals bright pairs of atoms. The new catalyst is regarded as an advance in efforts to produce and store clean energy through artificial photosynthesis. (Image credit: PNAS)
A structural characterization of a new iridium dinuclear heterogeneous catalyst reveals bright pairs of atoms. The new catalyst is regarded as an advance in efforts to produce and store clean energy through artificial photosynthesis. (Image credit: PNAS)
The scientists were able to leverage the new techniques in the assessment of single-atom catalysts and design a material platform to explore key and complex reactions, where more than one active site is required.
Wang said the researchers set out to find "what the smallest active and most durable heterogeneous catalyst unit for water oxidation could be. Previously, researchers have asked this question and found the answer only in homogeneous catalysts, whose durability was poor. For the first time, we have a glimpse of the potential of heterogeneous catalysts in clean energy production and storage."
The researchers also carried out X-ray experiments at Lawrence Berkeley National Laboratory's Advanced Light Source that allowed them to explore the iridium catalyst’s structure. X-ray absorption near edge structure (XANES) and X-ray absorption fine structure (EXAFS) were the two techniques used by the researchers in their measurements. From these experiments, the team was able to obtain critical evidence to gain insights into the new catalyst better.
Wang informed that the scientists were amazed by the simplicity and durability of their catalyst, along with high activity toward water oxidation, which is the desired reaction in photosynthesis.
Wang went on to say that the catalyst will be optimized further for practical use and areas of application where the catalyst can be used to new chemical transformations will be examined as the next steps of this research work.
Besides Wang and his research group at Boston College, researchers from the University of California, Irvine; Yale University; Tufts University; and Lawrence Berkeley National Laboratory, as well as the Chinese institutions Tsinghua University and Nanjing University have also contributed to the study.
The National Science Foundation and the U.S. Department of Energy, as well as scientific agencies in China, funded the research work.