Oct 18 2019
Researchers from the Tokyo Institute of Technology (Tokyo Tech) studied a material capable of splitting water molecules (H2O) to generate dihydrogen (H2) in the presence of sunlight. As H2 can serve as clean fuel, this study may offer a meaningful understanding for scientists working on clean energy production.
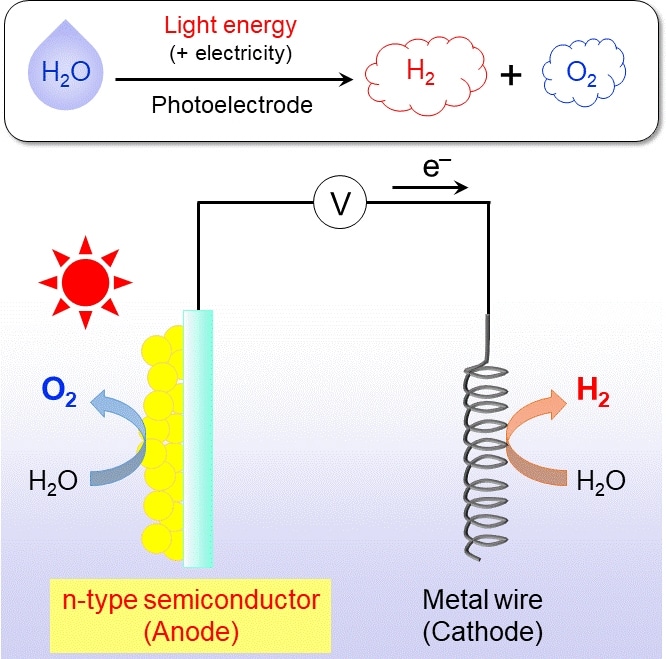 Photoelectrochemical water splitting. Water can be split to obtain H2 and O2 by applying a low voltage in a circuit like the one shown in the figure by using an appropriate photoanode material. The H2 generated can be used as clean fuel, meaning that photoelectrochemical water splitting is a way to harvest solar energy. (Image credit: Tokyo Institute of Technology)
Photoelectrochemical water splitting. Water can be split to obtain H2 and O2 by applying a low voltage in a circuit like the one shown in the figure by using an appropriate photoanode material. The H2 generated can be used as clean fuel, meaning that photoelectrochemical water splitting is a way to harvest solar energy. (Image credit: Tokyo Institute of Technology)
Due to the growing global concerns about the Earth’s state, optimizing the technology for producing alternative energy has become a subject of debate among scientists across the world.
Water splitting, one among the various methods being analyzed to produce clean energy, was found to be extremely potent. Specifically important was the process of photoelectrochemical water splitting, which involves using solar energy to split water molecules to obtain dihydrogen.
As it is possible to use dihydrogen as a clean fuel to produce electricity or for other machines, optimization of water splitting methods could be a promising way to minimize carbon emissions and lessen global warming.
One way of performing photoelectrochemical water splitting is by connecting a certain kind of semiconductor material, known as the photoanode, to a low-voltage source and a metal wire, which serves as the cathode.
In the presence of sunlight, water is split into its constituting atoms at the photoanode and cathode. These constituent atoms combine again to form the useful O2 and H2 as a byproduct. The important step here is to find stable, high-performance materials for the photoanode since the oxidation sub-step, involving the O2 formation, is the most difficult one.
However, most of the studies have focused on a group of photoanodes known as oxynitrides. These are unstable and degrade quite rapidly because, when exposed to light, they get oxidized.
To solve this problem, scientists from Tokyo Tech, under the leadership of Prof. Kazuhiko Maeda, used Pb2Ti2O5.4F1.2 (an oxyfluoride material), which does not undergo self-oxidation owing to its electronic properties.
Although this oxyfluoride was found to hold potential for several other applications, there have been no research works to reveal its photoelectrochemical performance as a photoanode for water splitting. The scientists analyzed this compound under different conditions of lighting and applied voltage.
They discovered that to use it as a photoanode, it is essential to alter its surface with other compounds. First, a titanium oxide (TiO2) layer must be deposited on the oxyfluoride’s surface to increase the photocurrent produced by the water splitting reaction.
Next, the efficiency of the photoanode can be significantly increased by additionally coating it with cobalt oxides (CoOx), which can penetrate through the cracks in the layer of TiO2 and stimulate the required reaction.
Post-modification of the photoanode with a water-oxidation promoter has proven to be indispensable to attaining stable performance in most cases.
Prof. Kazuhiko Maeda, Tokyo Institute of Technology
The scientists carried out many experiments to characterize the photoanode and its efficiency for splitting water under various conditions, like different voltages and pH values (which is a measure of water acidity), as well as different kinds of light. Their findings are meaningful and helpful to guide other scientists in the right direction.
So far, oxynitrides and similar compounds have been viewed as promising but difficult-to-handle materials for photoanodes because of their inherent instability to self-oxidation. Pb2Ti2O5.4F1.2 represents a long-awaited breakthrough in this regard.
Prof. Kazuhiko Maeda, Tokyo Institute of Technology
The process of splitting water will be vital to fulfill energy requirements without further damaging the environment. Research works similar to this one are important stepping stones to achieve the goals for a greener future.