Jan 22 2020
Scientists at the Center for Integrated Nanostructure Physics, within the Institute for Basic Science (IBS, South Korea), have developed a novel method to convert carbon dioxide (CO2) into pure carbon monoxide (CO) and oxygen (O2) by simply harvesting sunlight.
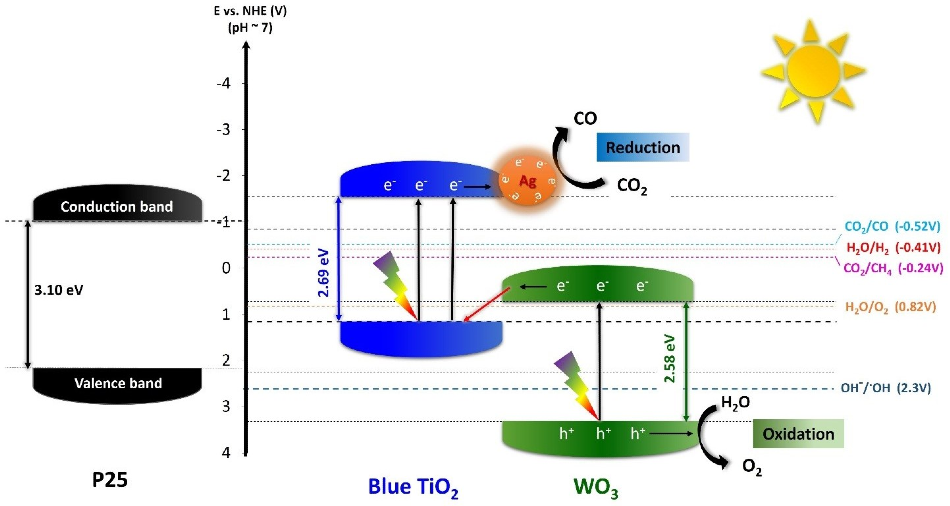
Image Credit: Institute for Basic Science.
This process does not leave behind any byproducts in water. The study has been published in the Materials Today journal.
While photosynthesis in green plants converts CO2 into sugars, the artificial photosynthesis described in the latest study can transform CO2 into O2 and pure CO as output. The pure CO can be subsequently employed for a wide range of applications in the pharmaceutical, electronics, chemical, and semiconductor industries.
It is essential to identify an optimal high-performance photocatalyst to promote photosynthesis by absorbing light, transforming CO2, and ensuring an efficient electron flow, which is important for the entire system.
Titanium oxide (TiO2) is a popular photocatalyst and has already attracted a great deal of attention in the areas of environmental protection and solar energy conversion fields because of its low cost, low toxicity, chemical stability, and high reactivity.
Although traditional TiO2 is capable of absorbing only UV light, the researchers at IBS had earlier reported two different types of blue-colored TiO2, or “blue titania,” nanoparticles that can absorb visible light because of a reduced bandgap of approximately 2.7 eV.
The nanoparticles were composed of ordered anatase/disordered rutile (Ao/Rd) TiO2 (known as HYL’s blue TiO2-I, as described in Energy & Environmental Science, 2016). They are also made of disordered anatase/ordered rutile (Ad/Ro) TiO2 (known as HYL’s blue TiO2-II, as described in ACS Applied Materials & Interfaces, 2019).
Here, rutile and anatase refer to two crystalline forms of TiO2, and the addition of irregularities, or disorder, in the crystal improves the absorption of both infrared light and visible light.
To efficiently convert CO2 into O2 and pure CO through artificial photosynthesis, the IBS team aimed to enhance the nanoparticles’ performance by integrating blue (Ao/Rd) TiO2 with other metals and semiconductors. Both metals and semiconductors can improve the oxidation of water to oxygen and simultaneously the reduction of CO2 into CO alone.
The researchers achieved optimal results with hybrid nanoparticles composed of tungsten trioxide (WO3), 1% silver (TiO2/WO3–Ag), and blue titania.
WO3 was selected due to its low cost, high stability, and the low valence band position with its 2.6 eV narrow bandgap. Silver was also added since it improves the absorption of visible light, by producing a collective oscillation of free electrons activated by light. Silver also provides high CO selectivity
The hybrid nanoparticles displayed around 200 times greater performance when compared to nanoparticles made of TiO2/WO3 without silver and TiO2 alone.
Using CO2 and water, the new hybrid catalyst generated pure CO as well as O2, without any byproducts such as methane (CH4) and hydrogen gas (H2). The evident quantum yield, that is, the ratio of the number of reacted electrons to the number of incident photons was 34.8%, and the speed of the reacted electrons was 2333.44 μmol g−1h−1.
The same kind of measurement was found to be lower for nanoparticles that lacked silver (2053.2 μmol g−1h−1), as well as for nanoparticles that have only blue TiO2 (912.4 μmol g−1h−1).