Nitrate ions are common pollutants that have been produced by agricultural run-offs, livestock waste, and municipal waste treatment systems.
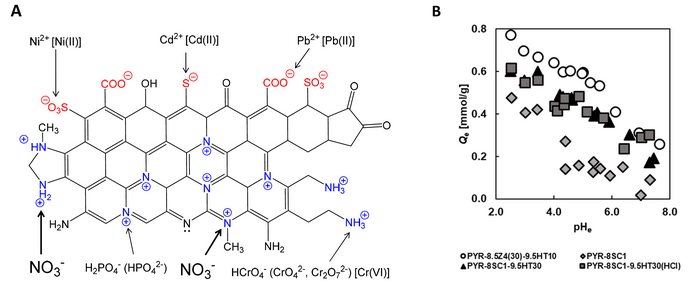
Researchers at Chiba University, Japan, prepared polyacrylonitrile (PAN)-based activated carbon fibers that are capable of removing nitrate ions from the environment. A. Adsorbent structure and its surface functional groups. Quaternary nitrogen (N-Q), including graphitic nitrogen, and aliphatic amine could be effective for the removal of anionic pollutants, such as nitrate, phosphate, chromium, and arsenic anions. B. A graph representing the influence of equilibrium solution pH (pHe) on the amount of NO3- adsorption. Image Credit: Motoi Machida from Chiba University, Japan
Even though a necessary amount of fertilizer is essential for growing food, nitrates could be detrimental when left to circulate in the ecosystem without being given proper treatment. For example, it can result in an algal bloom in water bodies, thereby decreasing the amount of dissolved oxygen in water, eventually posing a risk to aquatic organisms.
Also, nitrate ions have been linked to digestive cancers in adults and blood disorders in infants. Therefore, a simple and affordable method to eliminate nitrate ions from the ecosystem seems to be vital.
In recent years, scientists have made an attempt to try and test various materials that have the potential to eliminate nitrate ions through adsorption. A famous method is to make use of adsorbent materials, like activated carbon fibers (ACF).
The ACFs’ porous structure allows the initiation of an extensive range of functional groups that could be selected per the needs of adsorption. Also, they are simple to recover and reuse. Unluckily, there are a few drawbacks of ACF that impede its applications.
Professor Motoi heads the research on nitrate ion removal and gives insights into the problems that were experienced.
Carbonaceous materials like activated carbons are usually functionalized with negatively charged functional groups, such as carboxy, carbonyl or phenol, which show excellent adsorption performance for positively charged ions and organic pollutants but are ineffective against inorganic negatively charged ions.
Motoi Machida, Professor, Chiba University
Machida added, “So, we wanted to develop a durable ACF-based material that can efficiently adsorb nitrate ions and retain adsorption capacity for multiple uses.”
In a new discovery made available online on 10th October 2022 and published in SN Applied Sciences on 22nd October 2022, Professor Machida, together with Ms. Natsuho Sato and Associate Professor Yoshimasa Amano from Chiba University, has come up with a sodium carbonate-activated, polyacrylonitrile (PAN)-based ACF consisting high nitrogen content for nitrate ion removal.
For the adsorption property of the PAN-ACF to be increased, the team triggered it with sodium carbonate at a temperature of 800 °C. Furthermore, they heat-treated the fibers at 950 °C, thereby decreasing the nitrogen content. This increased the amount of quaternary nitrogen (N-Q), a positively charged functional group that has the potential for nitrate adsorption.
After the preparation process is done, the team performed tests to examine the nitrate ion adsorption dynamics of the material. The outcomes displayed that heat treatment increased the nitrate adsorption capacity by removing nitrate-repelling functional groups, like –COO– and –COOH. The PAN-ACF material displayed outstanding adsorption properties, with an adsorption capacity of 0.5 mmol/g nitrate ions from water.
Also, the team performed long-term degradation studies under various storage conditions to identify the best way of storing the material for improved reuse outcomes. They discovered that retaining the ACFs in a hydrochloric acid solution or under a vacuum kept them stable.
The adsorption capacity and degradation studies performed have disclosed that the material displayed a consistent adsorption rate at a pH of 4-5. This is a hopeful find since 4 to 5 is the pH range of groundwater.
Our new robust material can withstand treatments with boiling water, and strong acidic and basic solutions, thereby still easily regenerate with minimal loss in adsorption capacity.
Motoi Machida, Professor, Chiba University
On the whole, the new PAN-ACF presented in the study not only has the potential to treat environmental water by eliminating detrimental nitrate ions but also helps decrease the addiction to traditional plastic material-based ion exchange resins. This could be done by substituting them with more eco-friendly active carbon-based ion removers.
Journal Reference:
Sato, N., et al. (2022) Adsorption characteristics of nitrate ion by sodium carbonate activated PAN-based activated carbon fiber. SN Applied Sciences. doi.org/10.1007/s42452-022-05191-w.