AZoCleantech speaks with Stephanie Ribet and Prof. Vinayak Dravid from Northwestern University. Stephanie and Vinayak were part of a team that has developed a way to remove phosphate from aquatic systems and reuse it. The system hinges on a porous flexible membrane that can grab up to 99% of phosphate ions from polluted water. The team has named their system Phosphate Elimination and Recovery Lightweight (PEARL) membrane.
Can you tell our readers about the Phosphate Elimination and Recovery Lightweight (PEARL) membrane and how it was developed?
The Dravid group research is generally focused on remediation approaches that are rooted in science, while considering engineering, environmental and economic factors. Our team initially began working on water challenges by developing the OHM Sponge, a project led by a senior member of the Dravid group and co-author, Dr. Vikas Nandwana. The OHM sponge is a versatile, efficient and eco-friendly oil remediation technology that can be used repeatedly, minimizing hazardous waste and recovering the oil.
The OHM sponge project has taught us the power of "R" - Reduce, Reuse & Recycle to Restore (the environment). We wanted to apply these same principles to other environmental remediation challenges. Moreover, as we continued our water research, we looked to our local community. Northwestern University is located in the Great Lakes Region, and when we reflected on the critical problems in our neighborhood, nutrient pollution quickly surfaced as a key issue.
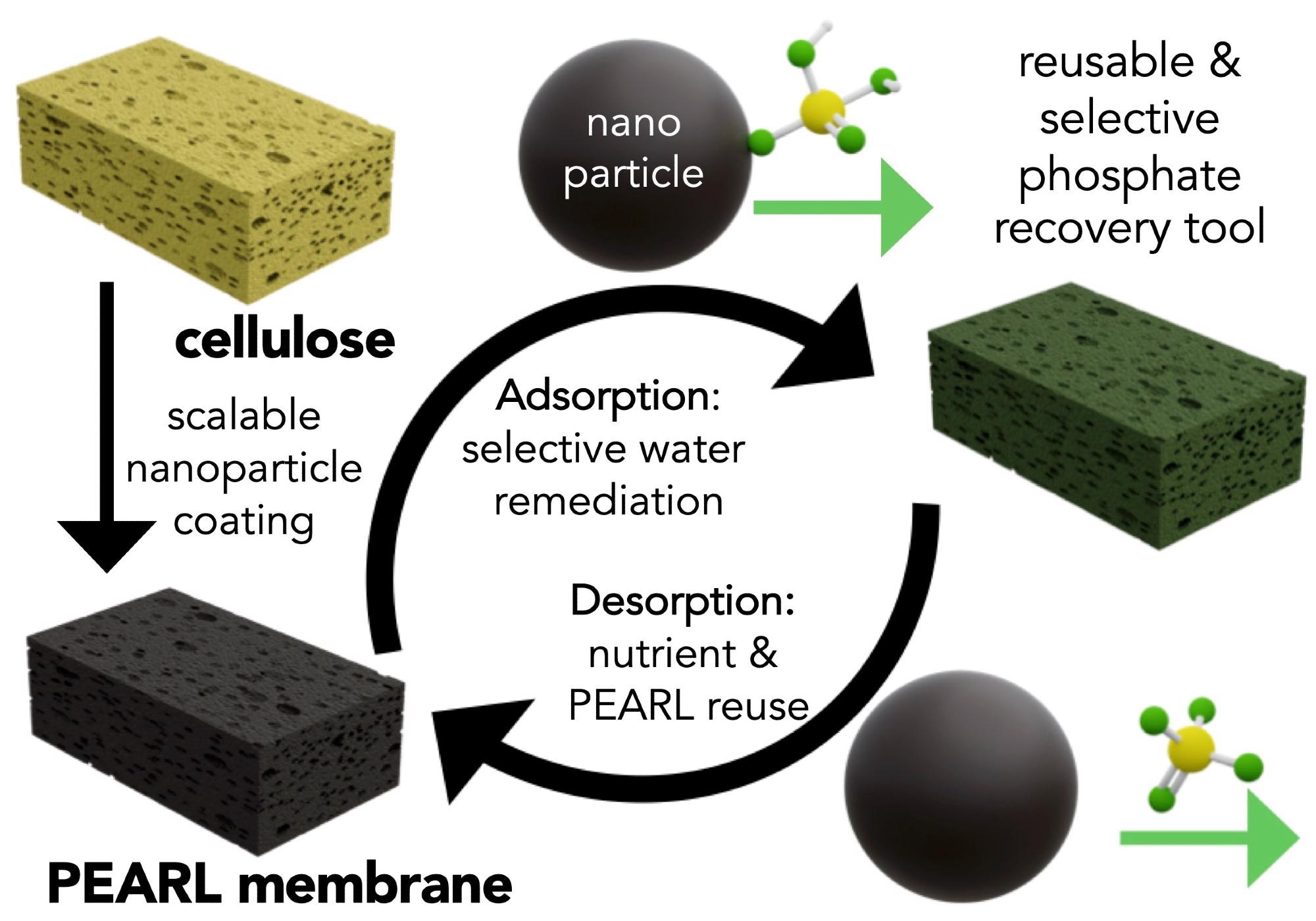
What was the process of testing the PEARL membrane?
We began by testing the performance of the PEARL membrane in small batches in a controlled environment at the lab scale. After our initial success, we experimented with scaling-up the material, using larger systems and less idea aqueous conditions. For example, we tested our material on water samples from the Metropolitan Water Reclamation District of Greater Chicago. With these complex samples containing a number of competing ions we were able to demonstrate the selectivity of our PEARL membrane.
In addition, we were interested in testing the PEARL membrane by understanding its "structure" (how it is built from the molecular scale to the large macroscopic visual scale), as this information can help with the improved design of this material. Led by co-author Dr. Roberto dos Reis, we could characterize the structure of the material down to its molecular scale building blocks with electron microscopy and a number of correlative techniques.
We learned, for example, that only a very small weight percent of the nanomaterials (~5-10 weight %) is necessary to turn cellulose into an effective environmental remediation platform, further enhancing the eco-friendly and efficient nature of the PEARL membrane. For these experiments we collaborated with teams at core facilities across Northwestern, including the NUANCE center.

Why is phosphate control so important in aquatic environments?
Anthropogenic hypereutrophication, namely excess nutrients released into natural bodies of water from human activity, can have substantial economic, environmental and public health consequences. In the presence of extra phosphates and nitrates, blue-green algae bloom, which block sunlight, produce toxins, and when they die, lead to the proliferation of bacteria. As bacteria consume dead matter, they use up oxygen, making an anoxic dead zone, prompting a positive feedback cycle. Hypereutrophication is expected to worsen with climate change, heightening the need for sustainable solutions to recover this anion before it causes environmental damage.
Your system works on recovering and reusing phosphate rather than eliminating it. Why is this important?
Phosphate rock is a non-renewable natural resource of limited supply, so it is important to recover these nutrients for further reuse. Given the shortage of this precious natural resource, it is sadly ironic that many of our lakes are suffering from hypereutrophication. In the past, our society has been better at reusing this anion before flushing it away. The scarcity of phosphate emphasizes the benefits of remediation approaches rooted in the concept of a circular economy.
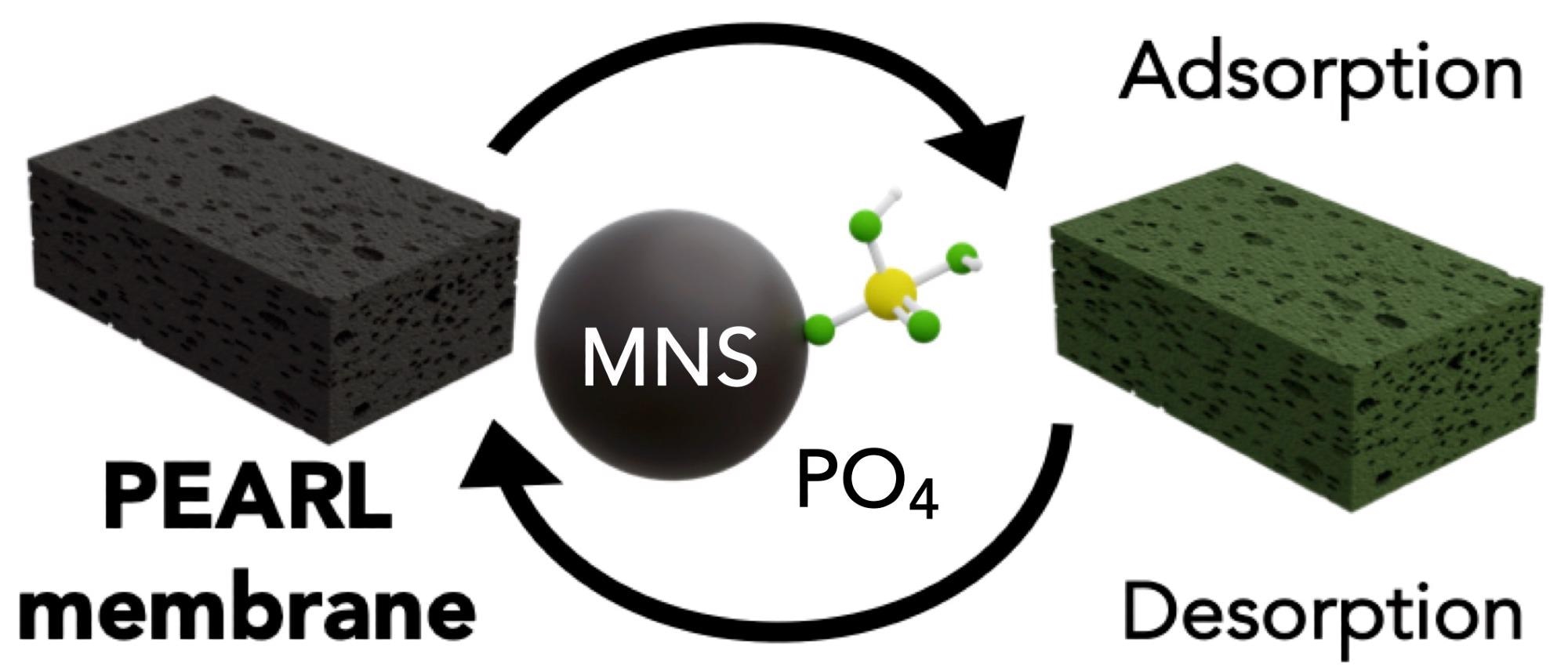
The PEARL membrane can be employed repeatedly; why is this so?
Making use of the pH sensitivity of phosphate sequestration, we can use mildly basic solutions to release phosphate anions. pH changes are a cheap and efficient method for sorption.
It was important to design a material where the nutrients could be adsorbed and desorbed at will. Not only does this facilitate nutrient reuse, but it also allows the PEARL membrane to be used for many cycles contributing to its environmental and economic promise.
You've described the breakthrough as a 'Swiss Army Knife' for tackling pollution. Why do you say this?
By modulating the nanocomposite coating, other types of contaminants can also be targeted. A combination of different coating materials can be used to remove (and recover) multiple pollutants at the same time.
The flexibility of these materials (both literally and figurately) means they can be deployed in a number of configurations, including as a sorbent pad, boom or fixed bed reactor, allowing this material to be further tailored to different environmental problems.
How can your platform approach be employed to tackle heavy metal pollution in waterways?
Led by co-author Benjamin Shindel, we are developing a similar composite material for the remediation of heavy metals. This ties into the "Swiss Army Knife" theme of our work.
What do you believe the role of materials science will be in tackling future environmental issues, particularly aquatic pollution?
We are plagued by countless environmental issues, and these grand challenges will require the coordination of solutions from many diverse teams. We believe there is a role for scientists in our field to make a contribution in these areas through the development of new materials. Aqueous pollution is a great example – we hope that the creation of new materials in concert with the engineering of new systems will lead to technologies that can have meaningful ecological impacts.

What's next for you and the team at Northwestern University?
It has been a lot of fun to work on these aqueous environmental remediation materials and most especially, to connect to and learn from others in the field. We expect continued growth in this area in the coming years, and we're looking forward to expanding our research. Our plan includes scaling-up this technology, extending our approach to other contaminants, and continuing our science-based exploration of the underlying mechanisms of pollutant removal. As we continue these efforts, we intend to ensure our research stays rooted in the power of "R" - Reduce, Reuse & Recycle to Restore (the environment).
Where can readers find more information?
Here are a number of links that provide more information:
https://vpd.ms.northwestern.edu/
https://news.northwestern.edu/stories/2021/06/phosphate-water-pollution-remediation/&fj=1
https://www.anthropocenemagazine.org/2021/06/a-nanoscale-solution-to-a-gigaton-problem/
https://www.tmj4.com/news/national/scientists-removing-phosphate-contamination-in-water-with-sponge-filtering
https://doi.org/10.1073/pnas.2102583118
https://doi.org/10.1021/acs.iecr.0c01493
https://www.nsf.gov/discoveries/disc_summ.jsp?cntn_id=300709&org=NSF&from=news
https://www.nature.com/scitable/knowledge/library/eutrophication-causes-consequences-and-controls-in-aquatic-102364466/
https://www.theatlantic.com/science/archive/2021/02/phosphorus-pollution-fertilizer/617937/
About Prof. Vinayak P. Dravid
 Professor Vinayak Dravid is the Abraham Harris Professor of Materials Science and Engineering at Northwestern University. His scholarly interests and methods revolve around "imaging" and characterization across diverse length-scales and disparate phenomena; from atoms to animals and from arts to astronomy. His research spans broad themes in nanotechnology as they relate to energy, environment and sustainability. He is the founding director of the NUANCE Center and SHyNE Resource, an NSF-NNCI network node of excellence in the Midwest. He has been recognized as one of the most highly cited researchers every year since 2016 by Clarivate Analytics.
Professor Vinayak Dravid is the Abraham Harris Professor of Materials Science and Engineering at Northwestern University. His scholarly interests and methods revolve around "imaging" and characterization across diverse length-scales and disparate phenomena; from atoms to animals and from arts to astronomy. His research spans broad themes in nanotechnology as they relate to energy, environment and sustainability. He is the founding director of the NUANCE Center and SHyNE Resource, an NSF-NNCI network node of excellence in the Midwest. He has been recognized as one of the most highly cited researchers every year since 2016 by Clarivate Analytics.
About Stephanie M. Ribet
 Stephanie Ribet is a Ph.D. Candidate in the VPD group in the Department of Materials Science and Engineering at Northwestern University. She works on developing nanocomposite materials for environmental remediation and characterizing them with microscopy and spectroscopy techniques.
Stephanie Ribet is a Ph.D. Candidate in the VPD group in the Department of Materials Science and Engineering at Northwestern University. She works on developing nanocomposite materials for environmental remediation and characterizing them with microscopy and spectroscopy techniques.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.