'Syngas' or 'synthesis gas' is a combination of hydrogen, carbon monoxide, small quantities of carbon dioxide and other trace gases. Normally derived from feedstocks, Syngas contains carbon, such as biomass, natural gas, heavy oil and coal. In creates synthetic natural gas and producing methanol or ammonia.
Syngas is produced as a result of gasification of a carbon-containing fuel to a gaseous product that has heating value. If syngas contains nitrogen, it must be separated, as both nitrogen and carbon monoxide have similar boiling points and it will be difficult to recover pure carbon monoxide through cryogenic processing.
Syngas has 50% of the energy density of natural gas and hence it can be burnt and used as a fuel source. Refinement of syngas before use allows CO2 to be stripped from the raw gas thereby enabling the use of CO2 in enhanced oil recovery processes.
Production of Syngas
The production of syngas includes the following phases:
The Heating Phase
The first step is gasification, a thermo-chemical process in which carbon-rich feedstocks like petro-coke, biomass or coal are converted into a gaseous compound consisting of carbon monoxide and hydrogen under high-heat, high pressure, oxygen depleted conditions.
Very high temperatures of gasification, normally between 800 and 1500°C (1472 and 2732°F) are achieved with the help of an external heat source or through partial oxidation of feedstock which releases heat.
The Reaction Phase
The feedstock reacts with carbon dioxide, water vapor and oxygen during gasification. The reaction is triggered by thermal decomposition for oxygen-rich materials.
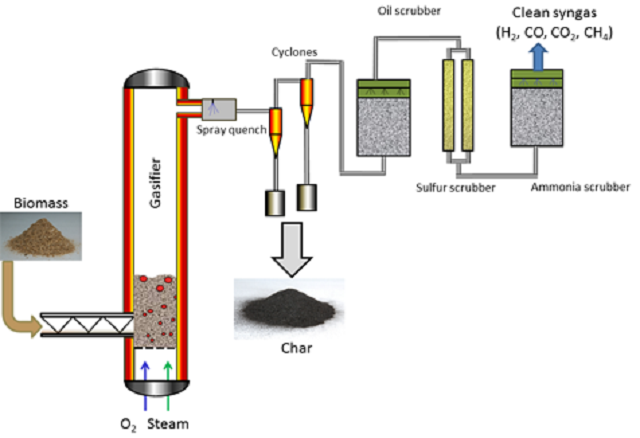
The process flow for Syngas production by gasification of biomass - Image Credits: www.iprt.iastate.edu
The Purification Phase
The gas obtained from gasification is raw and not clean enough to use. A purification process is carried out to eliminate impurities like ash, tar, sulfur compounds, methane, water vapor and carbon dioxide. The hydrogen-oxygen proportion is adjusted after purification depending on the applications of the synthesis processes.
The Catalytic Phase
Metals such as iron, manganese, cobalt, copper and new complex molecules are formed when the syngas is in contact with different catalysts. Scientists are experimenting with several catalysts to find new ways of creating already existing molecular combinations. In this way, it is possible to create eco-friendly fuels from syngas.
Syngas Cleanup and Conditioning
Raw syngas obtained from the gasification process needs to be cleaned to eliminate the contaminants such as mercury, chlorides, ammonia, sulfur, fine particulates and other trace heavy metals to protect downstream processes and to meet environmental emission regulations.
Syngas may be conditioned to adjust the hydrogen-to-carbon monoxide ratio based on the downstream process application.
Typical syngas cleanup and conditioning processes include the following:
- Removing bulk particulates using cyclone and filters
- Wet scrubbing for eliminating chlorides, ammonia and fine particulates
- Removing trace heavy metal and mercury using solid absorbents
- Water gas shift for adjusting hydrogen-to-carbon monoxide ratio
- Catalytic hydrolysis for converting carbonyl sulfide to hydrogen sulfide
- Acid gas removal for extracting sulfur-bearing gases and carbon dioxide.
SynGas Fermentation
Syngas fermentation is a microbial process in which syngas is used as a carbon and energy source, and then converted into chemicals and fuels with the help of microorganisms. Methane, butyric acid, acetic acid, butanol and ethanol are the main products of syngas fermentation.
Acetogens such as Clostridium carboxidivorans, Eurobacterium limosum, Butyribacterium methylotrophicum and Peptostreptococcus products are involved in the production of chemicals and fuels.
Some of the key benefits of syngas fermentation process include the following:
- High reaction specificity
- Low temperature and pressure
- Does not require a specific ratio of CO to H2
- Tolerate compounds having high sulfur content.
However, syngas fermentation has certain limitations such as inhibition of organisms, low volumetric productivity and gas-liquid mass transfer limitation.
Applications
Syngas can be used to produce a wide range of fertilizers, fuels, solvent and synthetic materials. Few examples are as follows:
- Steam for use in turbine drivers for electricity generation
- Nitrogen for use as pressurizing agents and fertilizers
- Hydrogen for electricity generation, use in refinery industry to extract more diesel and gasoline from crude oil and for a large variety of hydrogenation reactions where hydrogen is added to unsaturated hydrocarbons
- Ammonia for use as fertilizers and for the production of plastics like polyurethane and nylon.
- Methanol for the production of plastics, resins, pharmaceuticals, adhesives, paints and also as a component of fuels.
- Carbon monoxide for use in chemical industry feedstock and fuels
- Sulfur for use as elemental sulfur for chemical industry
- Minerals and solids for use as slag for roadbeds.
Production SynGas using Steam Reformer - Run Time - 3.00mins
Production SynGas using steam reformer
Spurces and Further Reading