Dec 4 2007
Research on hydrogen-fueled cars may be one step closer to application thanks to a new form of hydride discovered by scientists at the ESRF.
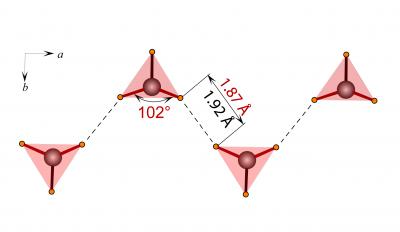 BH4 anions linked by short hydrogen-hydrogen contacts into chains in the crystal structure of the new phase. Source: Angewandte Chemie
BH4 anions linked by short hydrogen-hydrogen contacts into chains in the crystal structure of the new phase. Source: Angewandte Chemie
The material, lithium borohydride, is a promising energy storage system: it contains 18 weight percents of hydrogen, which makes it attractive for use in hydrogen-fueled cars. Its drawback is that it only releases hydrogen at quite high temperatures (above 300C). The team has found a new form of the compound that could possibly release hydrogen in mild conditions. This discovery was completely unexpected from the point of view of theoretical predictions.
Automotive industry regards hydrogen as a perspective energy carrier. If a good hydrogen storage material will be developed, the petrol in cars can be replaced by clean hydrogen energy. Five kilograms of hydrogen would take you as far as twenty liters of petrol. Today there are several compounds of interest, which are known to either store relatively large amounts of hydrogen or release it easily, but none do both in a way suitable for practical application.
Researchers at the Swiss-Norwegian experimental stations (beamlines) at the ESRF are currently studying several compounds of light elements with hydrogen and the different forms they take at different pressure and temperature. Lithium borohydride, LiBH4, is one of the compounds they study as it has a high weight content of hydrogen (18%). The new form of this compound, which scientists have just discovered, is promising because it appears to be unstable. Until today, all the known forms of this material are too stable, which means that they don’t let the hydrogen go. “This one is really unexpected and very encouraging”, says Yaroslav Filinchuk, the corresponding author of the paper.
In order to obtain new forms of lithium borohydride, the team applied to the sample pressures up to 200,000 bar. The pressure of 200,000 bar applied to LiBH4 in the ESRF experiment is about 80 times bigger than the pressure exerted on Earth's crust by Mount Everest (the latter is roughly equal to 2.5 kbar). Although impressive, this figure is not a record - much higher pressures still can be reached in the lab using the same diamond anvil cell technique, but this was not necessary for this experiment.
Diffraction of synchrotron light was used to determine arrangement of atoms in the resulting materials. In this way two novel structures of lithium borohydride were found. One of them is truly unprecedented and reveals strikingly short contacts between hydrogen atoms.
Combined experimental and theoretical efforts suggest that the new from of LiBH4 can release hydrogen at a lower temperature. Filinchuk explains that “the new form becomes even more attractive considering the fact it appears already at 10.000 bar, the pressure used by pharmaceutical companies to compress pellets”. The authors argue that this form can be stabilized by chemical substitutions even at ambient pressure. For now, the team’s next step is to apply chemical engineering to the compound to “freeze” the new form at ambient conditions and check whether it shows more favorable hydrogen storage properties than pure lithium borohydride.
Despite the fact that hydrogen is not well detected by X-rays in general, scientists managed to see it thanks to the high brilliance of the ESRF synchrotron light. Although theory failed to predict the novel structure, it fully supports this experimental finding. Therefore, this work presents a breakthrough in experimental studies of hydrogen-rich system, explains the failure of the previous theoretical predictions and suggests the novel form of the compound to be instrumental in obtaining improved hydrogen storage materials.
Synchrotron radiation was recently successfully applied to potential hydrogen storage materials and it turns out to be more useful than generally expected for so light systems. The team at the Swiss-Norwegian Beam Lines at the ESRF will continue to exploit and develop this at first glance unexpected union.