A group of scientists from Rice University and the Université catholique de Louvain has devised a technique to fabricate flexible components from waste silicon for use in rechargeable lithium-ion (LI) batteries.
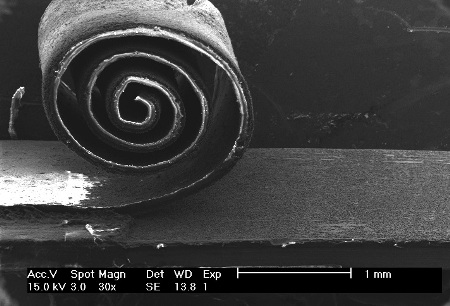
The silicon/copper/polymer composite can be rolled from its silicon substrate and leave a mask in place to begin anew the process of making another anode for a lithium-ion battery. (credit: Alexandru Vlad/Rice University).
Pulickel Ajayan, Rice University’s M. and Mary Greenwood Anderson Professor in Mechanical Engineering and Materials Science and of Chemistry, has used high-value but hard-to-recycle silicon to fabricate forests of nanowires.
Silicon absorbs 10 folds more lithium when compared to carbon generally utilized in LI batteries. However, the material breaks down rapidly as it gets expanded and contracted when it gets charged and discharged.
In the Proceedings of the National Academy of Science journal, Pulickel Ajayan and his colleagues have described a method to fabricate an anode from carefully arranged nanowires enclosed in an electrically conducting copper and an ion-conducting polymer electrolyte. The material provides nanowires the required space to expand and contract, thus increasing their efficacy. The electrolyte acts as a spacer between the cathode and the anode.
According to Ajayan, Converting waste into batteries must be a scalable technique. The scientists believe that their devices are a significant progress to develop next-generation flexible, efficient, low-cost batteries that is capable of conforming to any shape.
Co-lead authors, Arava Leela Mohana Reddy and Alexandru Vlad, created multiple anode/electrolyte composite layers from a single waste silicon wafer. They utilized colloidal nanosphere lithography to create a silicon corrosion mask by applying polystyrene beads dispersed in liquid over a discarded silicon wafer. The beads over the wafer assembled themselves into a hexagonal grid and remained set when contracted chemically.
The researchers then sprayed a thin gold layer and removed the polystyrene, leaving a fine gold mask comprising uniformly spaced holes over the wafer. They then placed the mask in a metal-assisted chemical etching, wherein the silicon dissolved where it contacted the metal, resulting in the formation of millions of uniformly spaced nanowires. They then applied a thin copper layer over the nanowires in order to enhance the lithium absorption capability of the wires and then infused the wires with an electrolyte.
The researchers then constructed a battery by integrating with a current collector and cathode on one side and a spray-on current collector on the other side. The battery demonstrated 150 mAh/g but decayed slightly over 50 charge/discharge cycles. The scientists are now involved in improving those qualities and assessing the anodes in regular battery configurations.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.