Nov 19 2013
Uranium mining for the nuclear industry causes immense environmental damage, which becomes more severe as reserves are depleted. The isolation of uranium from seawater would be a much more environmentally friendly alternative. In the journal Angewandte Chemie, American researchers have now introduced a process by which they can produce tailored, highly effective adsorption agents to do this job.
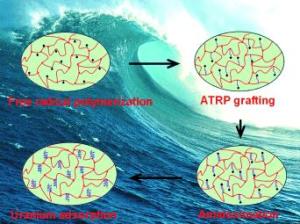
Because the concentration of uranyl ions in seawater is very low, adsorption agents used for this process must be particularly efficient. By carefully controlling the surface and pore structures, a team from Oak Ridge National Laboratory and the University of Tennessee has now been able to significantly increase both the rate and capacity of adsorption of a new polymer adsorbent.
Their success stems from a special polymerization technique. Sheng Dai’s team begins by producing a porous polymer framework based on the monomer vinylbenzyl chloride (VBC) with divinylbenzene (DVB) as a cross-linking agent. It is possible to vary the surface properties and pore volume of the product by changing the ratio of VBC to DVB. The interiors of the resulting frameworks contain many accessible chloride species that then serve as starting points for the next polymerization step, which is known as atom-transfer radical polymerization (ATRP). This reaction allows the researchers to grow polyacrylonitrile chains within the framework. The advantage of ATRP is that the length of the chains is highly controllable and uniform. In the final step, the polyacrylonitrile is converted to polyamidoxime because amidoxime groups bind well to uranyl ions.
Tests with simulated seawater resulted in distinctly higher and significantly faster uranium adsorption than with conventional, polyethylene-based adsorbents. Experiments showed that the adsorption capacity of the new adsorbent is strongly dependent on the density of amidoxime groups—a parameter that can be tailored by means of the pore size and the number of accessible chloride species in the original nanoporous framework. “These frameworks are the first example of ATRP initiators in which the initiator species is located within the nanoporous support network,” reports Dai. “This new process puts materials with tailored adsorption and surface properties within reach. The method can be used to produce a wide variety of polymer nanocomposites for applications including the removal of heavy-metal ions from solutions or novel catalysts.”