Nov 17 2014
Researchers at the UCLA Henry Samueli School of Engineering and Applied Science have developed a more efficient way to turn methanol into useful chemicals, such as liquid fuels, and that would also reduce carbon dioxide emissions.
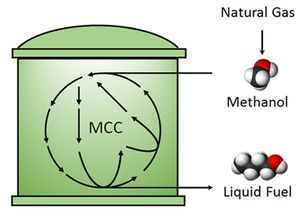 Conversion of methanol to liquid fuel. Credit: UCLA Engineering
Conversion of methanol to liquid fuel. Credit: UCLA Engineering
Methanol, which is a product of natural gas, is well-known as a common “feedstock” chemical — one that is processed into gasoline and other chemicals such as solvents, adhesives, paints and plastics. Using current methods, that processing requires high temperatures, high pressures, expensive catalysts, and typically results in the release of the greenhouse gas carbon dioxide into the atmosphere.
“The boom in natural gas in North America has been a game-changer in the energy and chemical industry, however challenges include how to make natural gas processing more efficient, how to lower expensive production costs, and to reduce emissions associated with fuel production,” said James C. Liao, UCLA’s Ralph M. Parsons Foundation Chair in Chemical Engineering, who was the principal investigator on the research. “Our new process offers solutions to one major step of those challenges.”
The UCLA team’s process synthesizes longer-chain molecules like butanol, which can be used for automobile fuel, under room temperature and ambient atmospheric pressures. The second key is that this process is “carbon efficient,” which means that no carbon is lost in this process (no carbon dioxide is released).
“That’s the beautiful part of this process, it completely conserves the carbon,” said Igor Bogorad, a UCLA Ph.D. student in bioengineering and co-author on the research paper. “Methanol is a largely untapped resource in the bioprocessing industry. The current dogma has been to find better uses of plant-derived sugars. However, methanol offers many advantages and its availability is expected to increase.”
Building off the success from their previous work published in Nature, the researchers modified the non-oxidative glycolysis pathway to utilize methanol instead of sugar. The new process, a biocatalytic pathway, is called “methanol condensation cycle.” They demonstrated this process using a set of purified enzymes and were able to synthesize ethanol and butanol (two- and four-carbon alcohols) from methanol.
The research was published in the Nov. 11 edition of the Proceedings of the National Academy of Sciences.
Tung-Yun (Tony) Wu, a project scientist with Liao’s metabolic engineering and synthetic biology laboratory, was a co-author and manager of the research. Other contributing authors included Chang-Ting Chen, Matthew Theisen, Alicia Schlenz and Albert Lam, also members of Liao’s lab.
While this research addresses a major step in converting methanol to liquid fuels, another major challenge remains in the conversion of methane (the major component in natural gas) to methanol.
“With our solution to methanol processing could be a major part of large-scale production that is much lower in cost than current standards,” said Liao, who is also the chair of the UCLA Department of Chemical and Biomolecular Engineering.
“We appreciate the financial support from the ARPA-e REMOTE program,” Wu said. “Without their support, this accomplishment would have been impossible.”
The research was supported by the ARPA-e award DE-AR0000430.