Oct 31 2015
An inexpensive method for generating clean fuel is the modern-day equivalent of the philosopher's stone. One compelling idea is to use solar energy to split water into its constituent hydrogen and oxygen and then harvest the hydrogen for use as fuel. But splitting water efficiently turns out to be not so easy.
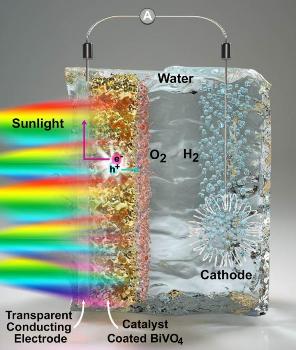 Splitting water into hydrogen provides a means of harvesting the hydrogen for fuel. This image depicts the water-splitting process in a light-sensitive electrode material (BiVO4), which UChicago and University of Wisconsin researchers investigated in an experimental and computational study. (CREDIT - Peter Allen)
Splitting water into hydrogen provides a means of harvesting the hydrogen for fuel. This image depicts the water-splitting process in a light-sensitive electrode material (BiVO4), which UChicago and University of Wisconsin researchers investigated in an experimental and computational study. (CREDIT - Peter Allen)
Now two scientists at the University of Chicago's Institute for Molecular Engineering (IME) and the University of Wisconsin have made an important contribution to the effort, improving the efficiency of the key processes and offering new conceptual tools that can be applied more broadly in the quest to split water with sunlight. Their results appeared online Oct. 26 in Nature Communications.
Kyoung-Shin Choi is a professor of chemistry at the University of Wisconsin, Madison, and an experimentalist. Giulia Galli is Liew Family Professor of Electronic Structure and Simulations at the IME and a theorist. Working together, the two found a way to increase the efficiency with which an electrode used for splitting water absorbs solar photons while at the same time improving the flow of electrons from one electrode to another.
Simulations allowed them to understand what was happening at the atomic level. "Our study will encourage researchers in the field to develop ways to improve multiple processes using a single treatment," said Choi. "So it's not just about achieving higher efficiency, it's about providing a strategy for the field."
Excited electrons
When building a sun-capturing electrode, scientists aim to use as much of the solar spectrum as possible to excite electrons in the electrode to move from one state to another, where they will be available for the water-splitting reaction. Equally important, but a separate problem entirely, the electrons need to move easily from the electrode to a counter-electrode, creating a flow of current. Until now, scientists have had to use separate manipulations to increase photon absorption and the movement of electrons in the materials they are testing.
Choi and postdoctoral researcher Tae Woo Kim found that if they heated an electrode made of the semiconducting compound bismuth vanadate to 350 degrees Celsius while flowing nitrogen gas over it, some of the nitrogen was incorporated into the compound.
The result was a notable increase in both photon absorption and electron transport. What was not clear was exactly how the nitrogen was facilitating the observed changes. So Choi turned to Galli, a theorist, to see if her simulations of the system could provide insight into what was going on.
Nitrogen's role
Galli and former graduate student Yuan Ping, now a post-doc at Caltech, found that the nitrogen was acting on the electrode in several ways. Heating the sample while flowing nitrogen gas is known to extract oxygen atoms from the bismuth vanadate, creating "defects." Galli's team found that these defects enhance the transport of electrons. But more interestingly, they found that the nitrogen that had been incorporated into the compound increased the transport of electrons independent of the defects.
Finally, that nitrogen lowered the energy needed to kick electrons into the state in which they were available to split water. This meant that more of the solar energy could be used by the electrode. "Now we understand what's going on at the microscopic level," said Galli. "So people can use these concepts --incorporation of a new element and new defects into the material -- in other systems to try to improve their efficiency. These are very general concepts that could also be applied to other materials."
It's axiomatic in science that experimentalists and theorists need one another. But it is actually not so common for them to collaborate from the beginning of a project as Galli's and Choi's teams have done. The two came together through a National Science Foundation initiative called the Center for Chemical Innovation -- Solar, led by Prof. Harry B. Gray of Caltech. The center fosters scientific collaborations aimed at coming up with a device to split water.
"We come from very different fields," said Galli. "But within this project we had a common focus and a common problem to solve. We also got to learn a lot from each other. The collaboration has been just wonderful."
Choi agreed. "When the theory and the experiment come together, performance improvement and atomic-level understanding of what is going on can be achieved simultaneously, she says. "That's the ideal outcome."