Jan 28 2016
Scientists have simplified the method to manufacture silicon solar cells, that convert sunlight to electricity, with high efficiency by adding a new mix of materials to a typical design.
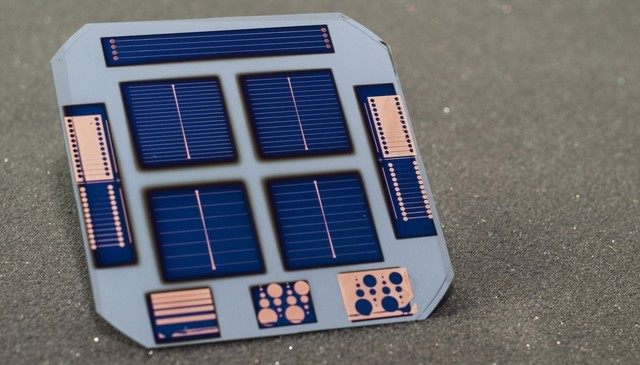 © PVLab-EPFL
© PVLab-EPFL
Published in Nature Energy, the research work is a collaborative effort between the Lawrence Berkeley National Laboratory (Berkeley Lab), EPFL, and the Australian National University (ANU).
The special mix of materials – that could also be helpful for semiconductor components – removes the need of 'doping', which controls the device’s semiconductor properties by introducing foreign atoms, and also leads to more complicated processing of the device and causes performance losses.
The solar-cell industry is driven by the need to reduce costs and increase performance. Conventional silicon solar cells use impurity doping, which brings about a number of limitations that make progress increasingly difficult. If you look at the architecture of the solar cell we made, it is very simple. That simplicity can translate to reduced costs.
James Bullock, Lead Author
Bullock participated in the study as a visiting researcher from the ANU at the U.S. Department of Energy's Berkeley Lab and UC Berkeley, where he will return soon as a postdoc.
Most of today’s solar cells employ crystalline silicon wafers. The wafer, and sometimes layers on the wafer, are doped with atoms that have spare electrons when they join silicon atoms, or generate electron deficiencies, referred as "holes." In both cases, doping improves electrical conductivity.
The two types of dopant atoms are needed at the electrical contacts to control the movement of electrons and holes in a solar cell, so that sunlight can be efficiently converted to an electrical current that flows out of the cell. But doping can result in performance losses too.
Solar cells made from crystalline silicon with doped contacts can exhibit more than 20% power-conversion efficiency, more than 20% of the sun's energy is converted into electricity. Silicon cells that have not been doped, have never had more than 14% efficiency. The new study showcases a dopant-free silicon cell – referred to as a dopant free asymmetric heterocontact (DASH) cell – with nearly 20% efficiency. The cell is made up of new materials, and involves a simple coating process for layers on the bottom and top of the device. The study shows that a solar cell can be created in just seven steps.
The research team used a crystalline silicon core (wafer), and applied a dopant-free amorphous silicon in layers for surface passivation. Ultrathin coatings of molybdenum oxide (moly oxide) were applied to the sun-facing surface of the solar cell, and lithium fluoride on the bottom surface. The two layers function as dopant-free contacts for holes and electrons.
Moly oxide and lithium fluoride exhibit extremely high and low work functions, respectively, which make them ideal for dopant-free electrical contacts. They were previously explored for organic devices, but they were not carefully explored by the crystalline Si solar cell community." Javey adds that the two materials are transparent, and possess complementary electronic structures, which are suited for solar cells.
Ali Javey, Professor, Electrical Engineering and Computer Sciences, UC Berkeley
Javey’s group discovered the role of moly oxide to act as a hole contact for crystalline silicon solar cells two years ago.
It has a lot of defects, and these defects are critical and important for the arising properties. These are good defects.
Ali Javey, Professor, Electrical Engineering and Computer Sciences, UC Berkeley
According to Stefaan de Wolf, team leader on crystalline silicon at EPFL Neuchâtel (Switzerland): “We have adapted the technology in our solar-cell manufacturing platform at EPFL, and found out that these moly oxide layers work extremely well when optimized and used in combination with thin amorphous layers of silicon on crystalline wafers, allowing amazing variations of our standard heterojunction approach.”
In the research, the team used lithium fluoride as a candidate material for electron contacts to crystalline silicon with a thin amorphous layer coating, to match the moly oxide hole contacts. The team used a room-temperature technique known as “thermal evaporation” for depositing lithium fluoride and moly oxide layers for the new solar cell. The researchers also wish to test other materials for their potential in the improvement of conversion efficiency of solar cells.
According to Javey, the material mix used in the study can be adapted for improving the performance of semiconductor transistors.
There's a critical need to reduce the contact resistance in transistors, so we're trying to see if this can help.
Ali Javey, Professor, Electrical Engineering and Computer Sciences, UC Berkeley