Feb 4 2016
Researchers from the USC Loker Hydrocarbon Research Institute have succeeded in generating fuel from thin air. This is the first time that researchers have directly transformed carbon dioxide, present in the air, into methanol. This conversion took place at a relatively low temperature.
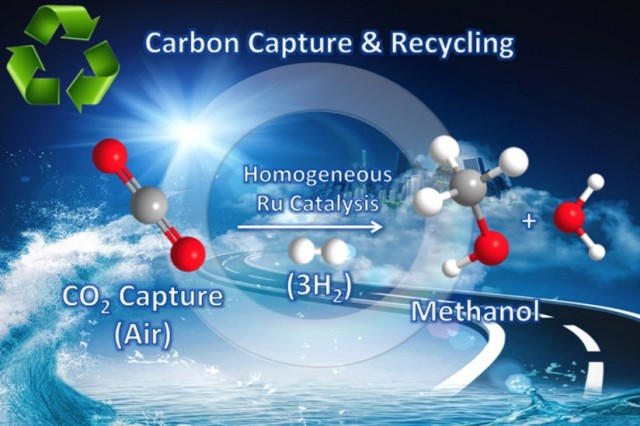 The carbon dioxide-to-methanol process (Illustration/Courtesy of Surya Prakash)
The carbon dioxide-to-methanol process (Illustration/Courtesy of Surya Prakash)
G.K. Surya and George Olah from the USC Dornsife College of Letters, Arts and Sciences, led the research work. The effort made by researchers is to concentrate on stabilizing the amount of carbon dioxide in the atmosphere by utilizing renewable energy, and at the same time converting greenhouse gases into a combustible resource, addressing global warming from two perspectives simultaneously. Methanol is used in internal combustion engines, as it is a clean-burning fuel. It is also used as a raw material for developing a number of petrochemical products, and as a fuel for fuel cells.
We need to learn to manage carbon. That is the future.
G.K Surya Prakash, Director, USC Loker Hydocarbon Research Institute
Prakash stated that an aqueous solution of pentaethylenehexamine (PEHA) was prepared, and a catalyst was added to help the hydrogen in the solution to capture CO2 under pressure. Air was bubbled into this aqueous solution, which was later heated. This resulted in converting 79% of the CO2 into methanol. The obtained methanol can be effortlessly distilled, even though water is present.
Industrial use
The researchers wish to utilize the energy the methanol has captured to source industrial usage, although it will take 5-10 years to apply it in the industrial field. The new process has been described in a paper in the Journal of the American Chemical Society.
Of course it won’t compete with oil today, at around $30 per barrel, but right now we burn fossilized sunshine. We will run out of oil and gas, but the sun will be there for another five billion years. So we need to be better at taking advantage of it as a resource.
G.K Surya Prakash, Director, USC Loker Hydocarbon Research Institute
Although carbon dioxide creates a tremendous impact on the environment, the National Oceanographic and Atmospheric Administration reports that the actual concentration is roughly 400 ppm, or 0.04% of the total volume. The amount of carbon dioxide is 23 times more than the amount of argon gas present in the atmosphere, yet CO2 makes up only less than 1% of the total volume.
Lower temperatures
The previously developed techniques needed a slower multistage process, high concentrations of CO2, and high temperatures, which implied that renewable energy sources were not capable enough to power the entire process.
The new system, devised by Prakash and Olah, operated at 125°C-165°C (257-359°F), and as a result reduced the decomposition of the catalyst, which occurred at 155°C (311°F). The new system used a homogenous catalyst that made it a quick “one-spot” process. The researchers proved the effectiveness of the catalyst by demonstrating the process five times.
Graduate student Jotheeswari Kothandaraman and senior research associates Alain Goeppert and Miklos Czaun of USC Dornsife also contributed to the study. The USC Loker Hydrocarbon Research Institute supported the research work.