Mar 4 2016
A multidisciplinary research team have developed a method to produce electric vehicles that are both carbon negative and carbon neutral. This means that the vehicle will be able to reduce the level of carbon dioxide in the atmosphere as they are driven.
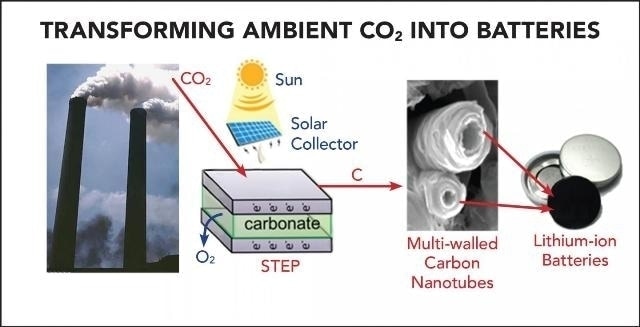 The Solar Thermal Electrochemical Process (STEP) converts atmospheric carbon dioxide into carbon nanotubes that can be used in advanced batteries (Credit: Julie Turner / Vanderbilt University)
The Solar Thermal Electrochemical Process (STEP) converts atmospheric carbon dioxide into carbon nanotubes that can be used in advanced batteries (Credit: Julie Turner / Vanderbilt University)
The scientists established the idea using electrodes made of carbon material regained from the atmosphere. Generally graphite-based electrodes are utilized in lithium-ion batteries, which power electric vehicles.
The process of changing carbon dioxide into batteries is detailed in the article published in the journal ACS Central Science. The article is titled “Carbon Nanotubes Produced from Ambient Carbon Dioxide for Environmentally Sustainable Lithium-Ion and Sodium-Ion Battery Anodes”.
The collaborative work of Cary Pint, Assistant Professor of Mechanical Engineering at Vanderbilt University and Stuart Licht Professor of Chemistry at George Washington University resulted in the unique combination of innovative battery technology and carbon dioxide conversion.
The researchers used a solar-driven process to produce carbon nanotubes, using the principle of conversion of carbon dioxide gas into carbon. They showed that the carbon nanotubes can be integrated into the lithium-ion batteries that power electronic instruments and electric automobiles. Nanotubes can also be used in low-cost sodium-ion batteries that are being developed for large-scale industrial applications like electric grid.
This approach not only produces better batteries but it also establishes a value for carbon dioxide recovered from the atmosphere that is associated with the end-user battery cost unlike most efforts to reuse CO2 that are aimed at low-valued fuels, like methanol, that cannot justify the cost required to produce them.
Cary Pint, Assistant Professor, Mechanical Engineering, Vanderbilt University
The study is based on a unique process called solar thermal electrochemical process (STEP), which is capable of producing carbon nanofibers from atmospheric carbon dioxide. This process was developed by the Licht team and published in the Nano letters journal in August 2015. STEP utilizes sunlight to provide the thermal and electrical energy required for splitting carbon dioxide into oxygen and carbon, and creates carbon nanotubes that have better flexibility, stability, conductivity and strength when compared to steel.
Our climate-change solution is two fold: (1) to transform the greenhouse gas carbon dioxide into valuable products and (2) to provide greenhouse gas emission-free alternatives to today’s industrial and transportation fossil fuel processes. In addition to better batteries other applications for the carbon nanotubes include carbon composites for strong, lightweight construction materials, sports equipment and car, truck and airplane bodies.
Stuart Licht, Professor, Chemistry, George Washington University
Pint is particularly interested in applying carbon nanomaterials for battery applications. The research labs worked together to establish that the multi-walled carbon nanotubes, created by the STEP process, can be used as positive electrodes in sodium-ion as well as in lithium-ion batteries.
The carbon anode was substituted by the nanotubes in lithium-ion batteries. This promoted a slight increase in battery performance, which further improved the rapid charging of the battery. Minute defects in the carbon were identified in sodium-ion batteries, which could be adjusted with the STEP process. Such defects can undo stable storage performance that is more than 3.5 times above than that of sodium-ion batteries, integrated with graphite-based electrodes. It was observed that carbon-nanotube batteries did not show any sign of wear, even after being subjected to regular charge-discharge cycles for 2.5 months.
Pint predicted that nearly 40% of a battery could be made from recycled carbon dioxide if any one of the two electrodes were made of carbon nanotubes. However, this estimation does not take into account the exterior protective packing, but STEP processes could be harnessed to create this packaging.
The Department of Energy reported that the average cost of lithium-ion batteries is $325 per kWh. With that estimation the researchers suggested that a kg of carbon dioxide has a value of nearly $18 as a battery material - six times more than when it is changed to methanol. This value will increase when moving from large-size batteries utilized in automobiles to smaller ones used in electronic devices. By integrating solar cells in batteries renewable power is generated which does not release any greenhouse gases. This approach is useful for stopping the present-day carbon cycle which poses a global threat in the days to come.
Licht also suggested that the STEP method could be combined to an electrical generator powered by natural gas. The electricity, heat, and a concentrated carbon dioxide source produced by this generator could amplify the performance of the STEP process. The oxygen produced during the process could be sent back to the generator to increase its combustion efficiency and offset the electricity consumed in the STEP process. This would result in the development of a fossil fuel electrical power plant with zero emission of carbon dioxide.
Imagine a world where every new electric vehicle or grid-scale battery installation would not only enable us to overcome the environmental sins of our past, but also provide a step toward a sustainable future for our children. Our efforts have shown a path to achieve such a future.
Cary Pint, Assistant Professor, Mechanical Engineering, Vanderbilt University
Other coauthors of the paper include Rachel Carter, graduate student in mechanical engineering at Vanderbilt; Jiawen Ren, postdoctoral associate in chemistry at George Washington University; Anna Douglas, graduate student in the interdisciplinary materials science program at Vanderbilt; and Matthew Lefler, graduate student in chemistry at George Washington University.
The NSF Graduate Research Fellowship grant 1445197 and the National Science Foundation grants 123-732 and 1505830 partly supported the research.