Aug 26 2016
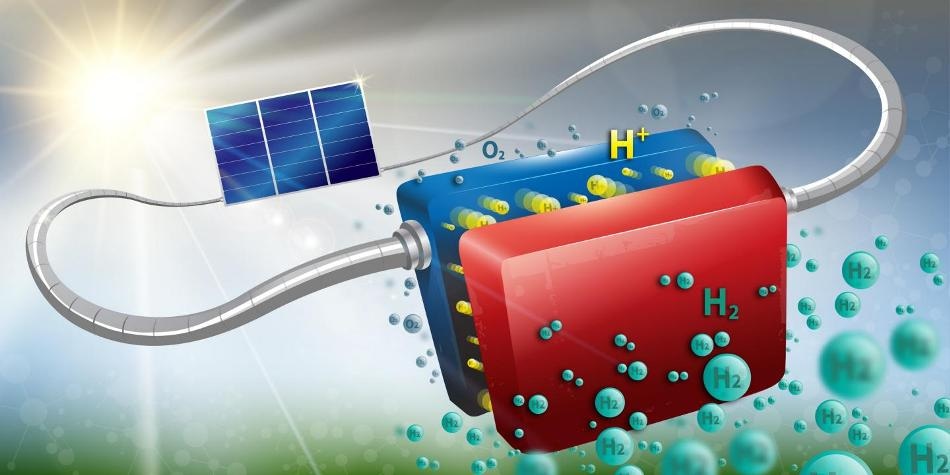 The device is able to convert solar energy into hydrogen at a rate of 14.2 percent, and has already been run for more than 100 hours straight. (CREDIT: Infini Lab / EPFL)
The device is able to convert solar energy into hydrogen at a rate of 14.2 percent, and has already been run for more than 100 hours straight. (CREDIT: Infini Lab / EPFL)
Even when the sun isn't shining, solar energy can still be utilized if it is converted into hydrogen through a process known as water electrolysis. This process aims to split water molecules into hydrogen and oxygen using the electric current generated from the solar panel.
This clean hydrogen can then be used as a fuel or can also be stored for electricity production in the future.
Despite the existence of many hydrogen-producing technologies that have provided promising results in the laboratory, the process is still complicated. This is because they are either expensive, stoo unstable, or require further enhancement to be used on a commercial and large scale.
EPFL and CSEM researchers are now involved in developing an effective and robust system using components that have already been well established in the industry. Their prototype consists of three new-generation, interconnected, crystalline silicon solar cells that are fixed to an electrolysis system that does not depend on rare metals.
In this device, conversion of solar energy into hydrogen takes place at a rate of 14.2%, and the device has already been tested for over 100 hours at a stretch under test conditions. As far as the performance is concerned, this is a world record for hydrogen production without utilizing any rare metal and also for silicon solar cells. A high level of stability is also offered by this system.
Enough to Power a Fuel Cell Car Over 10,000 km Every Year
This technique that outshines the existing techniques in terms of cost efficiency, lifespan, performance, and stability has been published in the Journal of The Electrochemical Society.
A 12-14 m2 system installed in Switzerland would allow the generation and storage of enough hydrogen to power a fuel cell car over 10,000 km every year.
Christophe Ballif, Co-Author
High Voltage Cells Have an Edge
The key is to use a 'hybrid' crystalline-silicon solar cell that is based on heterojunction technology, and also to utilize the existing components to the maximum possible extent. A sandwich structure developed by the researchers using layers of amorphous silicon and crystalline silicon permits higher voltages.
As a result, just three of these interconnected cells can conveniently generate an almost ideal voltage that is required for electrolysis to take place. A widely available catalyst that is made from nickel is required for the electrochemical part of the process.
"With conventional crystalline silicon cells, we would have to link up four cells to get the same voltage," says co-author Miguel Modestino at EPFL."So that's the strength of this method."
A Stable and Economically Viable Method
When it comes to lifespan, cost and performance, the new system is unique.
We wanted to develop a high performance system that can work under current conditions. The heterojunction cells that we use belong to the family of crystalline silicon cells, which alone account for about 90% of the solar panel market. It is a well-known and robust technology whose lifespan exceeds 25 years. And it also happens to cover the south side of the CSEM building in Neuchâtel.
Jan-Willem Schüttauf, Researcher, CSEM
Standard heterojunction cells were used by the researchers to prove this concept. By using the best cells of that type the researchers hope to achieve a performance above 16%.