Oct 19 2016
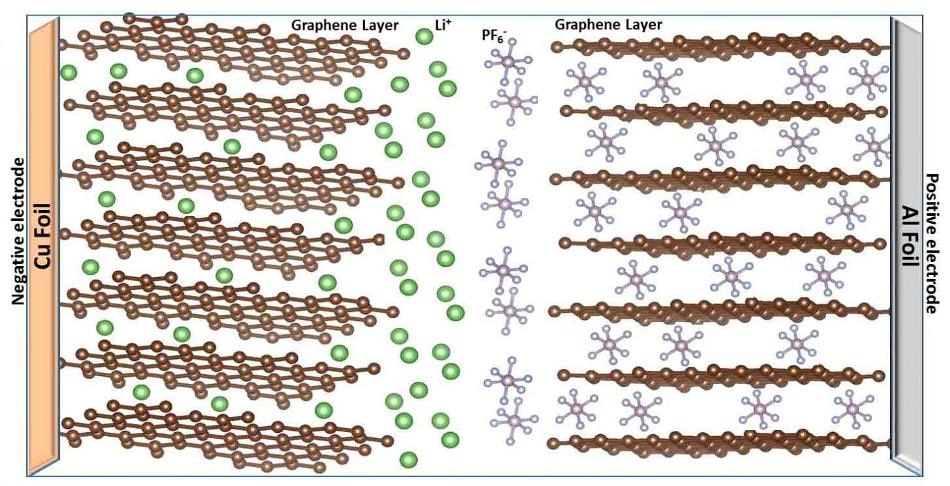 In dual-ion batteries, upon charging, anions migrate into the lattice of the positive electrode and cations move into the structure of the negative electrode. (CREDIT: (Image courtesy of Oregon State University))
In dual-ion batteries, upon charging, anions migrate into the lattice of the positive electrode and cations move into the structure of the negative electrode. (CREDIT: (Image courtesy of Oregon State University))
Oregon State University chemists have identified that one or more organic compounds that belongs to a family of pollutants can potentially contribute to an important advancement in manufacturing cost-effective and reliable batteries.
Such batteries will be of specific use in storing electricity derived from some clean energy systems. The inability to conveniently and cost-effectively store energy harnessed from the sun and wind, which is extremely intermittent and variable, has been one of the main obstacles in using these forms of energy.
Although compressed air facilities or pumped hydro systems possess all of the alternative energy storage capacity of this kind, they have some limitations. Scientists say that, there is tremendous demand for energy storage solutions. Energy storage solutions like smart grid and micro-grid uses that are suitable for community storage and are also modular are specifically in demand.
A new advancement that was published in the ACS Energy Letters shows that at least one, and potentially more compounds known as polycyclic aromatic hydrocarbons (PAHs) possess the ability to function as a durable, high-performance and low-cost cathode in dual-ion batteries.
These batteries comprise of a solid PAH as the cathode, and a carbon electrode as the anode. They eliminate the need for rare or costly metal elements that are usually used in batteries.
PAHs, which are generally known as pollutants, are obtained products of combustion (anything that is obtained from an automobile exhaust, coal-burning power plant or a campfire). When inhaled, these PAHs present significant concerns as toxins and carcinogens.
In this study, scientists discovered that at least one PAH compound, known as coronene, in its safe and crystallized solid form can be used to build a high-functioning electrode material with promising behavior in dual-ion batteries
Prior to this work, PAHs were not considered stable when storing large anions. We found that coronene crystalline solid, a PAH, can lose electrons and provide a good capacity of anion storage while being structurally and chemically stable. Coronene had good performance as an electrode and the ability to have a very long cycle life, or the number of charges and discharges it can handle.
Xiulei (David) Ji, Assistant Professor Oregon State University
Ji was the recipient of a 2016 National Science Foundation CAREER Award, the most prestigious award for junior faculty.
Ji stated that omitting the use of metals in the electrodes is a great advantage for dual-ion batteries; as a result the batteries become more sustainable. Although graphite cathodes can perform the same action, researchers have not been able to use them for the past two decades because they function at levels that are hostile to the non-aqueous solvents in the electrolyte. However, coronene eliminates this limitation and helps in significantly improving the sustainability and the maintenance cost of a stationary battery system.
The researchers showcased the potential of coronene in this study, and also added that other PAH compounds may also possess similar potential.
The researchers highlight that this research has paved way to a totally new concept in battery construction, which could change an unwanted pollutant in to a safe and valued product.
The preliminary collaborators of this project in OSU’s Department of Chemistry include lead author and graduate student Ismael Rodriguez-Perez, and professors Michael Lerner and Rich Carter.
The American Chemical Society Petroleum Research Fund supported this project.