Dec 21 2016
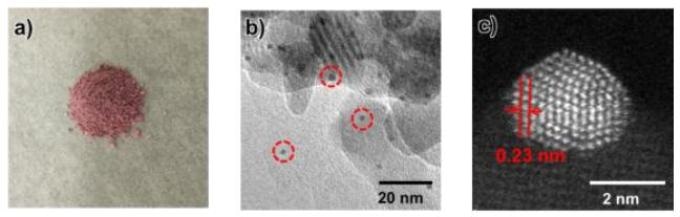 This image shows a) Gold nanoparticle catalyst (Au/HAP-NC), b) Images of gold nanoparticles (black dot in red circle) through Transmission Electron Microscope, c) Annular Dark Field of gold nanoparticles through Scanning Electron Microscope.(Credit - Osaka University)
This image shows a) Gold nanoparticle catalyst (Au/HAP-NC), b) Images of gold nanoparticles (black dot in red circle) through Transmission Electron Microscope, c) Annular Dark Field of gold nanoparticles through Scanning Electron Microscope.(Credit - Osaka University)
Hydrogen gas has potential as an alternative energy source to overcome the dependence on carbon-based fuels.
It also has the benefit of producing only water when it reacts with oxygen. However, the problem is that hydrogen is highly reactive and flammable, so it has to be carefully handled and stored.
Factors like risk of explosion, water sensitivity, and difficulty of control of hydrogen-generation limit typical hydrogen storage materials.
It is possible to efficiently produce hydrogen gas from organosilanes, some of which are suitably non-toxic, air-stable, and economical. Catalysts that can efficiently create hydrogen from organosilanes are desired with the ultimate goal of attaining safe, economical hydrogen production in high yield.
Preferably, the catalyst should also function at room temperature under aerobic conditions with no additional energy input.
Recently, a research team guided by Kiyotomi Kaneda and Takato Mitsudome at Osaka University have developed a catalyst that enables efficient environmentally friendly hydrogen production from organosilanes.
The catalyst is made up of gold nanoparticles with a diameter of about 2 nm supported on hydroxyapatite. Using chloroauric acid using glutathione as a capping agent, the catalyst was synthesized to prevent nanoparticle aggregation, causing the formation of small size of gold nanoparticles. Glutathione-capped gold nanoparticles were then adsorbed on hydroxyapatite and glutathione was removed by calcinations later.
The nanoparticle catalyst was then added to solutions of different organosilanes by the team to measure its ability to stimulate hydrogen production. The nanoparticle catalyst exhibited the maximum turnover frequency and number realized to date for hydrogen production catalysts from organosilanes.
For instance, the nanoparticle catalyst converted 99% of dimethylphenylsilane to the corresponding silanol within nine minutes at room temperature, discharging an equimolar quantity of hydrogen gas at the same time.
Significantly, the catalyst was recyclable with no loss of activity. On/off switching of hydrogen production was accomplished using the nanoparticle catalyst because it was possible to easily separate it from its organosilane substrate using filtration. As the nanoparticle size decreased, the activity of the catalyst increased.
A prototype portable hydrogen fuel cell made up of the nanoparticle catalyst and an organosilane substrate was fabricated. The fuel cell produced power in air at room temperature and could be switched on and off as required.
Images of the catalyst after use in the fuel cell looked like those of the unused catalyst, signifying that the hydroxyapatite-supported nanoparticle catalyst was able to resist aggregation.
Production of hydrogen from economical organosilane substrates under ambient conditions without extra energy input signifies a positive advance towards accomplishing the use of hydrogen as a green energy source.