Jan 10 2017
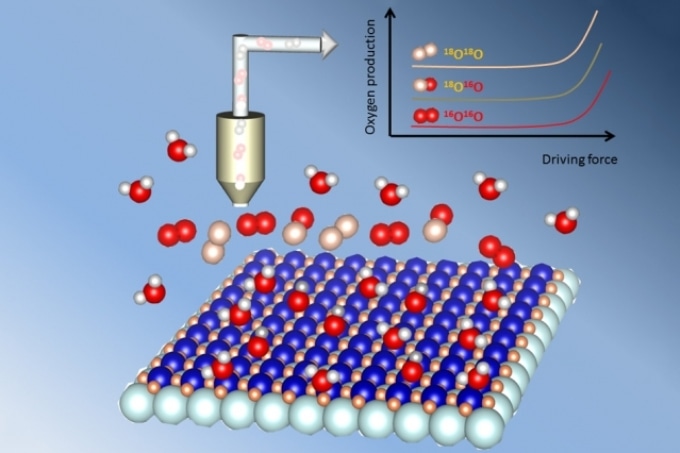 New research shows that when metal oxide (flat array of atoms at bottom) is used as a catalyst for splitting water molecules, some of the oxygen produced comes out of the metal oxide itself, not just from the surrounding water. This was proved by first using water with a heavier isotope of oxygen (oxygen 18, shown in white), and later switching to ordinary water (made with oxygen 16, shown in red). Courtesy of the researchers
New research shows that when metal oxide (flat array of atoms at bottom) is used as a catalyst for splitting water molecules, some of the oxygen produced comes out of the metal oxide itself, not just from the surrounding water. This was proved by first using water with a heavier isotope of oxygen (oxygen 18, shown in white), and later switching to ordinary water (made with oxygen 16, shown in red). Courtesy of the researchers
Chemical reactions that release oxygen in the presence of a catalyst, called oxygen-evolution reactions, are a vital part of chemical energy storage processes, including ammonia production, electrochemical carbon dioxide reduction, and water splitting.
The kinetics of this type of reaction are usually low, but compounds known as metal oxides can comprise of catalytic activities that differ over several orders of magnitude, with a few exhibiting the highest rates reported so far. The physical origins of these observed catalytic activities are yet to be well-understood.
A team at MIT has demonstrated that in some of these catalysts, oxygen does not only come from the water molecules surrounding the catalyst material; some of the oxygen comes from within the crystal lattice of the catalyst material itself.
The latest issue of the journal Nature Chemistry features a paper on the findings of this research. This paper was presented by recent MIT graduate Binghong Han PhD ’16, postdoc Alexis Grimaud, Yang Shao-Horn, the W.M. Keck Professor of Energy, and six others.
Grimaud states that the research focused on analyzing how water molecules are divided in order to generate oxygen molecules and further studying the factors that limit the reaction rate. For instance, more efficient energy storage and retrieval can be obtained by increasing those reaction rates, so establishing where the bottlenecks may be in the reaction is a vital step toward such improvements.
Metal oxides are the catalysts typically used to foster the reactions, and the team wanted “to be able to explain the activity of the sites [on the surface of the catalyst] that split the water,” Grimaud says.
Earlier debates have focused on the question of whether some oxygen gets stored within the crystal structure of the catalyst and then contributes to the total oxygen output. However, this issue remained unresolved by previous work.
Most researchers assumed that only the active sites on the material’s surface were taking any part in the reaction. But this team discovered a way of directly quantifying the contribution that could be coming from within the bulk of the catalyst material, and highlighted that this was an important part of the reaction.
The team used the isotope oxygen-18, a special “labeled” form of oxygen, which makes up just a tiny fraction of the oxygen in ordinary water. The team collaborated with Oscar Diaz-Morales and Marc T. Koper at Leiden University, Netherlands, to initially expose the catalyst to water made almost entirely of oxygen-18. The catalyst was then placed in normal water containing the more common oxygen-16.
After testing the oxygen output from the reaction, the researchers used a mass spectrometer that can directly measure the varied isotopes based on their atomic weight in order to show that a considerable amount of oxygen-18, which cannot be accounted for by a surface-only mechanism, was indeed being released.
Completion of the work took a longer time as the measurements were tricky to carry out. Diaz-Morales “did many experiments using the mass spectrometer to detect the kind of oxygen that was evolved from the water,” says Shao-Horn, who has joint appointments in the departments of Mechanical Engineering and Materials Science and Engineering, and is a co-director of the MIT Energy Initiative’s Center for Energy Storage.
With this knowledge and the detailed theoretical calculations highlighting how the reaction takes place, the team state that they can now discover ways of tuning the electronic structure of these metal-oxide materials in order to increase the reaction rate.
The team discovered that the quantity of oxygen contributed by the catalyst material differs considerably depending on the accurate chemistry or electronic structure of the catalyst. Oxides of varied metal ions on the perovskite structure showed lesser or greater effects, or even none at all.
Based on the volume of oxygen output generated from within the bulk of the catalyst, “you observe a well-defined signal of the labeled oxygen,” Shao-Horn says.
One surprising finding was that varying the alkalinity or acidity of the water made a huge difference to the reaction kinetics. Han says that increasing the pH of the water will result in improving the rate of oxygen evolution in the catalytic process.
These two earlier unidentified effects, the bulk material’s participation in the reaction, and the influence of the pH level on the reaction rate, which were discovered only for oxides with record high catalytic activity, “cannot be explained by the traditional mechanism” used to demonstrate oxygen evolution reaction kinetics, Diaz-Morales says. “We have proposed different mechanisms to account for these effects, which requires further experimental and computational studies.”
I find it very interesting that the lattice oxygen can take part in the oxygen evolution reactions. We used to think that all these basic electrochemical reactions, related to proton membrane fuel cells and electrolyzers, are all taking place at the surface,” but this work highlights that “the oxygen sitting inside the catalyst is also taking part in the reaction.
Ib Chorkendorff, Professor of Physics, Technical University of Denmark
These findings, he says, “challenge the common way of thinking and may lead us down new alleys, finding new and more efficient catalysts.”
The team also included Wesley Hong PhD ’16, former postdoc Yueh-Lin Lee, research scientist Livia Giordano in the Department of Mechanical Engineering, Kelsey Stoerzinger PhD ’16, and Marc Koper of the Leiden Institute of Chemistry, in the Netherlands. The Skoltech Center for Electrochemical Energy, the Singapore-MIT Alliance for Research and Technology, the Department of Energy, and the National Energy Technology Laboratory supported this work.