Feb 2 2017
 (Credit: https://www.olcf.ornl.gov/)
(Credit: https://www.olcf.ornl.gov/)
Researchers have been looking for better battery materials ever since Alessandro Volta, an Italian physicist, invented the first battery out of a stack of zinc and copper disks separated by moistened cardboard.
Lithium-ion batteries are longer-lasting, lighter, and functional under a wider range of temperatures compared to standard batteries, and they power everything from cell phones to electric cars to aircraft carriers. Their universal use makes their efficiency, stability, and safety important for consumers and businesses alike.
However, one of the key challenges faced by researchers dealing with battery components is finding innovative, nonflammable materials for the electrolyte. The electrolyte is the key battery component that transports lithium ions during charging and discharging, and transfers the energy that enables a battery’s use.
Now, researchers are searching for electrolytes that are stable as well as conductive to lithium ions, a property that lithium-ion batteries need in order to maintain efficiency during charge cycles.
At the US Department of Energy’s (DOE’s) Oak Ridge National Laboratory (ORNL), a research team headed by the California Institute of Technology’s (Caltech’s) Thomas Miller used the Cray XK7 Titan supercomputer to find promising electrolyte materials and predict materials that could improve the performance of lithium-ion batteries.
The researchers used Titan to run hundreds of simulations, each containing thousands of atoms, on potential new electrolytes. The study helped researchers to identify novel electrolytes with promising properties for lithium-ion conduction.
Miller, a professor of chemistry at Caltech and the principal investigator on the project, said that a leadership-class supercomputer was necessary to meet the goals of the project because the simulations ran on timescales ranging from a femtosecond (one quadrillionth of a second) to a microsecond (one millionth of a second), spanning nine orders of magnitude.
These calculations are extremely demanding in terms of computational resources. We are dealing with - from a molecular perspective - very big systems and long timescales
Thomas Miller, Professor, Caltech
According to Miller, the team has to quickly describe complex materials to screen across numerous candidate electrolytes. Luckily Titan, part of the Oak Ridge Leadership Computing Facility (OLCF), a DOE Office of Science User Facility at ORNL, allowed them to do just that.
Electrodes, Electrons, and Electrolytes
All batteries consist of an electrolyte, a solid or liquid that insulates the flow of electrons but promotes the flow of ions between the cathode and anode, which are the two electrodes that carry electrical current. Electrons in the battery travel through a circuit out of the battery and power a tool on the way to the cathode, the positive electrode of the battery.
The positively charged ions (the lithium ions) travel across the electrolyte to the cathode when the electrons leave the anode. This process goes on until the reactants are exhausted (meaning the battery loses its charge) or the circuit is disconnected.
The cycle can be reversed in rechargeable batteries, with the lithium ions diffusing back to the anode during the time of charging. The lithium ions are conserved during the time of charging and discharging, diffusing back and forth between the electrodes by an hourglass-like mechanism where the lithium ions are similar to the grains of sand that adjust direction when the reaction reverses.
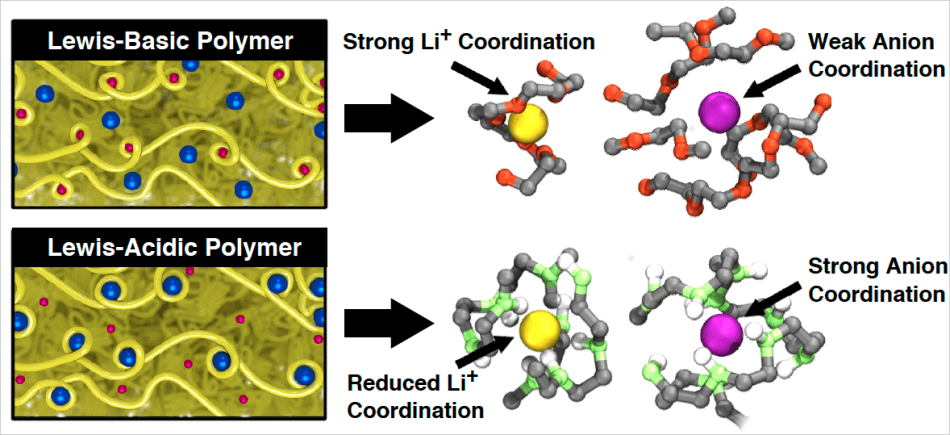
Lithium-ion batteries typically feature liquid electrolytes, however, the new research is concentrating on polymeric electrolytes, which are known to be less flammable, less volatile, and more stable. Proteins, rubber, and plastics are polymers, or long series of sequentially repeating molecules, that are used in various technologies due to their reliability and diverse functionality.
The polymers that we are studying are made of one molecular unit that’s repeated tens of thousands of times.
Thomas Miller, Professor, Caltech
Historically, polyethylene oxide (PEO), a versatile polymer with many applications in both science and medicine, has been the best polymer electrolyte for lithium-ion batteries. With the addition of lithium salts to polymers like PEO, these polymers can be used as solid polymer electrolytes.
The salt contains lithium cations, positively charged ions that get shuttled backward and forward in the battery, and some negatively charged anions in order to balance the charge.
A perfect electrolyte is one that readily dissolves and then conducts lithium ions. The difficulty with PEO is that it poorly conducts lithium ions compared to liquid electrolytes, meaning the ions move slowly to the cathode reducing the current the battery can generate.
It also conduct the anions too quickly. Although the anions are effective for balancing the charge of lithium ions, their quick conduction creates a loss in battery voltage.
Miller’s research team used this knowledge to look for more efficient polymers. Brett Savoie, a postdoctoral fellow at Caltech, considered that reversing the typical solid polymer electrolyte dynamic would assist Miller’s team to find more perfect polymers. “You want to conduct the positive lithium ions,” Miller said. “You don’t want to conduct the negative ions in the salt.”
The research team started to look for polymers that would conduct lithium ions more rapidly than PEO. The researchers used high-performance computing to create the chemically specific dynamic bond percolation model, a coarse-grained simulation approach that was developed and validated by the team, to screen electrolyte materials primarily based on short molecular dynamics trajectories. Initially, they screened a set of 500 different classes of polymers to find the better conductors of lithium ions.
One specific group of polymers meets the requirement.
Simulating Lewis-Acidic Polymers
Lewis-acidic molecules hold a positive charge and effectively interact with anions. Miller’s team used Lewis-acidic polymers to design simulations for electrolytes hoping that they would slow down anion conduction. According to Miller, these polymers have not been studied or simulated experimentally.
The researchers found that, within the simulation, this class of polymers conducted the anions more slowly compared to PEO and conducted the positive lithium ions more rapidly. Since Lewis-acidic chemical groups’ positive regions are contained in a small quantity of space and their negative regions are spread over a large quantity of space, they provide the positive lithium ions with more opportunities to dissolve, said Miller.
It was known that Lewis-acidic molecules slowed down anions. What was surprising here was that by using a purely Lewis-acidic system, we also sped up the lithium.
Brett Savoie, Postdoctoral Fellow, Caltech
The simulations revealed that these polymers may be able to produce an eight-fold increase in desired lithium conduction and a marked reduction in the unwanted anion conduction. This might be - given the traditionally slow pace of finding new polymer materials - a very large jump.
The researchers tracked the molecular evolution on timescales, ranging from a femtosecond to a microsecond. The capability to span this huge variety of timescales was made possible with Titan, which may compute at a rate of 27 petaflops, or 27 quadrillion calculations per second.
LAMMPS, an open-source classical molecular dynamics code, was used by the team to run the simulations in order to examine several dozen combinations of polymer and salt under different salt concentrations. At a time, almost 400 simulations were run in parallel, with each simulation using 1 GPU and 16 CPUs.
Each simulation contained about 3,000 atoms that are periodically replicated in three-dimensional space in order to create the effect of a bulk polymer material, with a specific concentration of ions per unit of periodic replication.
Supercomputers Conducive to Polymer Screening
Although Miller’s team continues to screen for promising polymer sequences with the aim of completing its 5,000th candidate electrolyte towards the end of this year, the project has already made the identification of polymers possible and this may favor lithium-ion conduction.
These new polymers are exciting because they seem to overcome some of the main problems with other polymer materials. The predictions indicate that these polymers might exhibit a substantial increase in conductivity. It would be a tremendous improvement from the current lithium-ion conductivity that PEO affords.
Thomas Miller, Professor, Caltech
The researchers are continuing to run their simulations on Titan under an Innovative and Novel Computational Impact on Theory and Experiment award, and for this they have been allotted 40 million core hours.
According to Miller, next-generation supercomputers like the OLCF’s Summit, which is scheduled to come online in 2018, will significantly expand their research capabilities and allow the team to examine even larger regions of chemical space.
“With faster computers we’ll be able to do this with even better accuracy,” Miller said. “We’ll also be able to look at more polymers more reliably and on longer timescales. Improved computers are going to rapidly accelerate the pace of discovery for materials of this kind.”
The research was supported by the National Science Foundation. Researchers also made use of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility at Lawrence Berkeley National Laboratory.