Feb 22 2017
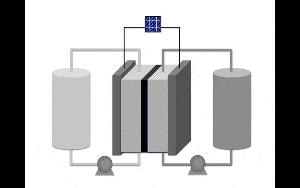 A diagram of a redox flow battery. An energy source, in this case a solar panel, provides the energy to the central cell to charge the battery. The charge is held in tanks of electrolytes that are pumped back into the cell to discharge the battery. PHOTO CREDIT: Image by Sharmila Samaroo/University of Michigan.
A diagram of a redox flow battery. An energy source, in this case a solar panel, provides the energy to the central cell to charge the battery. The charge is held in tanks of electrolytes that are pumped back into the cell to discharge the battery. PHOTO CREDIT: Image by Sharmila Samaroo/University of Michigan.
Solar utilities need a way to store extra charge for emergencies, as the sun does not shine every day. The case is similar with wind power, as the wind may not always blow.
Electrical grids require large batteries that can store the power transmitted from solar and wind installations until it is required in order to derive full advantage of renewable energy. Although some of the current technologies look very appealing, they are short-lived and inefficient for the electrical grid.
Participating in the U.S. Department of Energy’s Joint Center for Energy Storage Research, chemists from the University of Michigan and University of Utah, predict a better future for a type of battery for grid storage, which is called a redox flow battery.
The team has developed a charge-storing molecule - about 1,000 times more stable than current compounds - by employing a predictive model of molecules and their properties. Their results are reported in the Feb. 21 issue of the Journal of the American Chemical Society.
“Our first compound had a half-life of about eight-12 hours,” says U chemist Matthew Sigman, referring to the time period in which half of the compound would decompose. “The compound that we predicted was stable on the order of months.”
Not Your Ordinary Battery
Electricity needs to be either used as it is generated, sold back to the electrical grid, or stored in batteries, for a typical residential solar panel customer. Although lithium ion batteries or deep-cycle lead batteries are available, they present challenges for use on the electrical grid.
Electrical charge is stored and released by chemicals contained in the batteries. However, redox flow batteries are different from car batteries or cell phone batteries. For energy storage, these batteries use two tanks, which are separated by a central set of inert electrodes.
The tanks store the solutions consisting of molecules or charged atoms, which are known as anolytes and catholytes. Depending on whether electricity is provided to the battery or extracted from it, the anolytes and catholytes store and release charge when the solution “flows” past the electrodes.
If you want to increase the capacity, you just put more material in the tanks and it flows through the same cell. If you want to increase the rate of charge or discharge, you increase the number of cells.
Melanie Sanford, Chemist, University of Michigan
Solutions containing vanadium are currently used in redox flow batteries. Vanadium is expensive, and requires safe handling because of its potential toxicity. Formulating the batteries is a challenge, as molecules storing more charge tend to be less stable, and losing charge and quickly decomposing.
Molecular Bumper Cars
Sanford began working with Sigman and U electrochemist Shelley Minteer through the U.S. Department of Energy’s Joint Center for Energy Storage Research (JCESR), which is an Energy Innovation Hub devoted to creation of next-generation battery technologies.
Potential electrolyte molecules were developed and tested by Sanford’s lab, which also sought to use predictive technology to help design better battery compounds. Electrochemistry expertise was brought in by Minteer, and Sigman’s computational method using the structural features of a molecule was employed to predict its properties.
Such an approach is also commonly employed in drug development to predict the properties of candidate drugs.
The team realized that when two molecules interacted with each other a candidate compound was decomposed.
These molecules can’t decompose if they can’t come together. You can tune the molecules to prevent them from coming together.
Melanie Sanford, Chemist, University of Michigan
A bumper or deflector was placed to shield around the candidate molecule by tuning an important parameter of those molecules, a factor describing the height of a molecular component.
The paper reports that the most exciting anolyte is based on the organic molecule, pyridinium. The stability of this anolyte is improved as it does not contain metals and is intended to be dissolved in an organic solvent.
This anolyte offers the best combination of stability and redox potential, which is concerned with the amount of energy storage, though other compounds showed longer half-lives.
Sharing Skills to Build Batteries
Currently, Sigman, Minteer and Sanford are trying to identify a catholyte to pair with this anolyte and future molecules. Determining a framework for enhancing battery components is an important first step, although other engineering issues remain to be solved in the development of a new redox flow battery technology.
It’s a multipart challenge, but you can’t do anything if you don’t have stable molecules with low redox potentials. You need to work from there.
Melanie Sanford, Chemist, University of Michigan
The success of the team until now has been the application of this structure-function relationship toolset, commonly used in the pharmaceutical industry, to battery design. “We bring the tools of chemists to a field that was traditionally the purview of engineers,” Sanford says.
The Joint Center for Energy Storage Research, a Department of Energy Innovation Hub supported by DOE’s Office of Science, funded the project.