Mar 24 2017
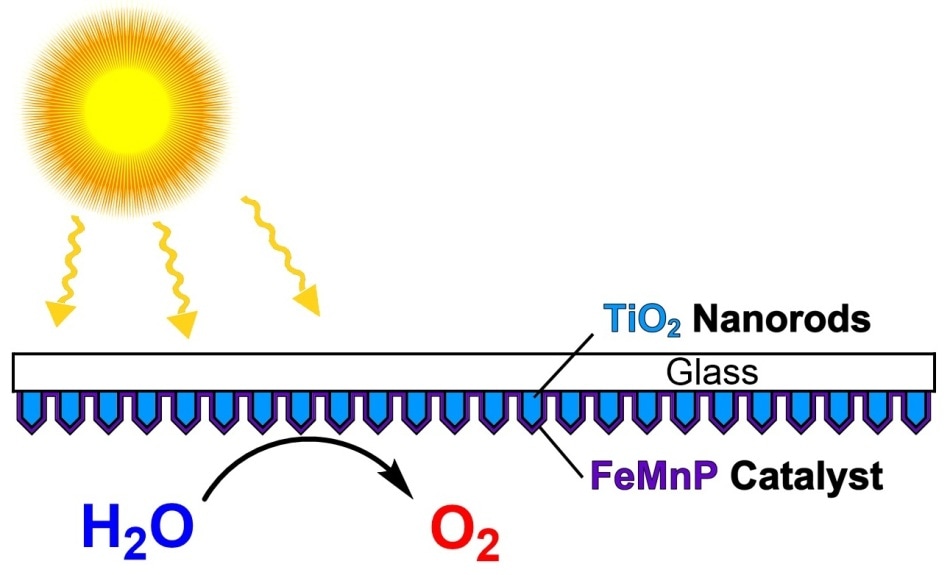 Scientists at Rice University and the University of Houston created a catalyst from three elements � iron, manganese and phosphorus � and then coated it evenly onto an array of titanium dioxide nanorods to create a highly efficient photoanode for artificial photosynthesis. Click on the image for a larger version. Courtesy of the Whitmire Research Group
Scientists at Rice University and the University of Houston created a catalyst from three elements � iron, manganese and phosphorus � and then coated it evenly onto an array of titanium dioxide nanorods to create a highly efficient photoanode for artificial photosynthesis. Click on the image for a larger version. Courtesy of the Whitmire Research Group
An efficient, simple-to-manufacture oxygen-evolution catalyst has been created by Rice University scientists. This catalyst matches well with semiconductors for solar water splitting, the changing of solar energy to chemical energy in the form of oxygen and hydrogen.
The lab of Kenton Whitmire, a Rice professor of chemistry, collaborated with researchers at the University of Houston and studied that growing a layer of an active catalyst directly on a light-absorbing nanorod array’s surface created an artificial photosynthesis material capable of splitting water at the full theoretical potential of the light-absorbing semiconductor with sunlight.
Water is split into oxygen and hydrogen with an oxygen-evolution catalyst. Extensive research focuses on discovering a clean renewable source of hydrogen fuel, however the technology is yet to be commercialized.
The team from Rice discovered a way to merge phosphorus, manganese and iron, three of the most abundant metals, into a precursor that can be directly deposited onto any substrate without causing any damage.
The researchers reported that the precursor was placed into its custom chemical vapor deposition (CVD) furnace by the lab in order to demonstrate the material. The precursor was then used to coat an array of light-absorbing, semiconducting titanium dioxide nanorods. The combined material called a photoanode showed outstanding stability while attaining a current density of 10 milliamps per square centimeter.
The results are published in two new studies. The first, dealing with the creation of the films, appears in Chemistry: A European Journal. The second, appearing in ACS Nano, details the creation of photoanodes.
Whitmire explained that the catalyst is grown from a molecular precursor custom designed to produce it upon decomposition, and the process is scalable. Iron, manganese and phosphorus (FeMnP) were combined by the Rice lab into a molecule that transforms into a gas when vacuum is applied. This gas decomposes to coat a surface with the FeMnP catalyst when it encounters a hot surface via CVD.
The researchers declare that their film is “the first heterobimetallic phosphide thin film” developed from manganese, iron and phosphorus that starts out as a single precursor. The resulting films comprise of hexagonal arrays of atoms that, until now, was only visible at temperatures more than 1,200 °C. The Rice films were developed at 350 °C in 30 minutes.
Temperatures above 1,200 C destroy the semiconductor array. But these films can be made at low temperatures, allowing them to evenly coat and interact with the photo absorber and create a hybrid electrode.
Kenton Whitmire, Professor, Rice University
The three-dimensional arrays of titanium dioxide nanorods were coated with the metallic-looking film by the researchers. The composite material displayed potential as a high-surface-area semiconductor for photoelectrochemical cells.
Maximum contact between the two is allowed by growing the transition metal coating directly onto the nanorods, Whitmire stated. “That metallic, conductive interface between the semiconductor and the active catalytic surface is key to the way this device works,” he said.
The film is also made up of ferromagnetic properties, in which the magnetic moments of the atoms align in the same direction. The film has a low Curie temperature, which refers to the temperature at which magnetic properties of specific materials need to be induced. The researchers stated that this could be useful for magnetic refrigeration.
With the technique being established, Whitmire said it will indeed be much easier now to examine hybrid catalysts for a number of applications, including refrigeration, energy conversion and petrochemical production.
It seems like when it rains, it pours. We spent a very long time putting everything together, and now all of a sudden there are too many things to do.
Kenton Whitmire, Professor, Rice University
Andrew Leitner, a postdoctoral researcher from Rice is lead author of the Chemistry: A European Journal paper. Co-authors are graduate students Desmond Schipper and Binod Kumar Rai; former postdoctoral researcher Jing-Han Chen; graduate alumnus Adam Colson, presently an assistant professor of chemistry at Boise State University; and Emilia Morosan, a professor of physics and astronomy, of chemistry and of materials science and nanoengineering, all at Rice; and Irene Rusakova, a senior research scientist at the University of Houston.
Lead authors of the ACS Nano paper are Schipper and Zhenhuan Zhao, a postdoctoral fellow at the University of Houston. Co-authors are Leitner and University of Houston graduate students Fan Qin, Zhiming Wang, Kamrul Alam and Shuo Chen; undergraduate student Lixin Xie, research professor Dezhi Wang; Zhifeng Ren, the MD Anderson Chair Professor; and Jiming Bao, an associate professor of electrical and computer engineering.
The research was supported by the National Science Foundation, the Robert A. Welch Foundation, the Gordon and Betty Moore Foundation and Rice University.