Mar 29 2017
 Credit: Institute of Basic Science
Credit: Institute of Basic Science
Scientists from the Institute of Basic Science (IBS) observe the ultrafast real-time bonding of lithium ions with the solvents in the same process that takes place during the charge and discharge of lithium batteries. With this observation, the researchers conclude on the need for a new theory.
What goes on inside lithium rechargeable batteries is not completely understood, although most of the electronic devices, such as laptops, mobile phones and electric vehicles use them. Researchers from the Center for Molecular Spectroscopy and Dynamics, within the IBS, have been successful in observing the real-time ultrafast dynamics of lithium ions with femtosecond time resolution (1/1,000,000,000,000,000 of a second). What happens during the process of charging and discharging are explained by these observations: an important development for the advanced batteries with better performance. Published on Nature Communications, this study describes the interactions between electrolytes and lithium ions, where the former are organic molecules surrounding the ions and conducting electricity. The study concluded that the established theory on ion diffusion in lithium rechargeable batteries is not fully correct.
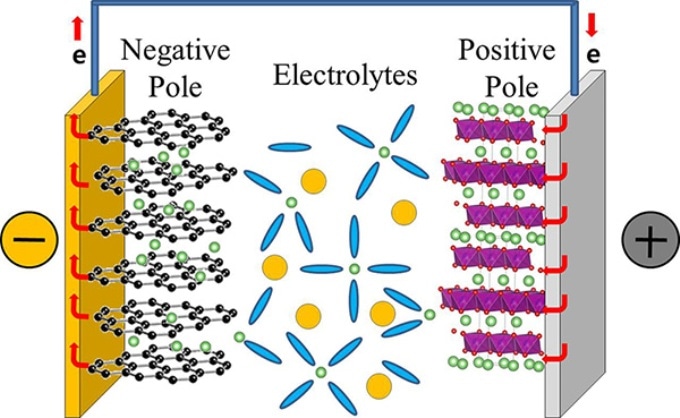
During the charging process of a typical commercial lithium rechargeable battery, there is movement of lithium ions, dissolved in electrolytes, from the positive to the negative pole of the battery. They move in the reverse direction, when the battery is in use. The movement of lithium ion dictates the performance of the lithium rechargeable battery, and determines the rapidity of the charging and discharging processes.
However, lithium ions do not migrate on their own. They are surrounded by electrolytes, which assist the inter-pole movement. The electrolytes in the existing lithium rechargeable batteries are typically made up of a mixture of dimethyl carbonate (DMC), ethylene carbonate (EC), and diethyl carbonate (DEC) in equal concentration. It has been thought, until now, that lithium ions mainly get associated with EC to form the so-called "solvation sheath" or "solvation shell", while DEC and DMC act like lubricants, and only facilitate the movement of these shells between the batteries' poles. While most of the earlier studies dealt with the static properties of the bond between lithium ions and electrolytes, the present investigation focuses on the dynamics of the bonding. In a motion picture, a series of still images are quickly displayed one after another to create the effect of movements. Similarly, IBS scientists took quick images to examine the creation and rupture of these bonds. While movies are usually filmed and displayed at 24 still-images per seconds, in this study, the measurement images were captured at time intervals of femtoseconds.
Aided by two-dimensional infrared spectroscopy, the scientists took measurements of how lithium ions bind to the oxygen atoms of the DEC. It was found that these bonds are broken and formed in a time period lasting 2-17 picoseconds (1/1,000,000,000,000 of a second). Similar timescale has been observed for DMC also. This means that DEC and DMC, other than being just "lubricants", are also a part of the solvation shell together with EC, and may be playinga key role in moving the lithium ions to the battery's pole.
It was believed that EC makes a rigid shell around lithium ions during the migration between electrodes. However, this study shows that the solvent shell is not that rigid, it is constantly restructured during the ion transport. For this reason, revising the existing lithium ion diffusion theory is inevitable.
Professor CHO Minhaeng
The research team is now focusing on a follow-up study in order to present a new theory of the lithium ion diffusion process. For this purpose, a new ultra-high-speed laser spectroscopy instrument is being built so as to observe the chemical reaction and also film it on top of the electrodes of the rechargeable batteries.