Apr 3 2017
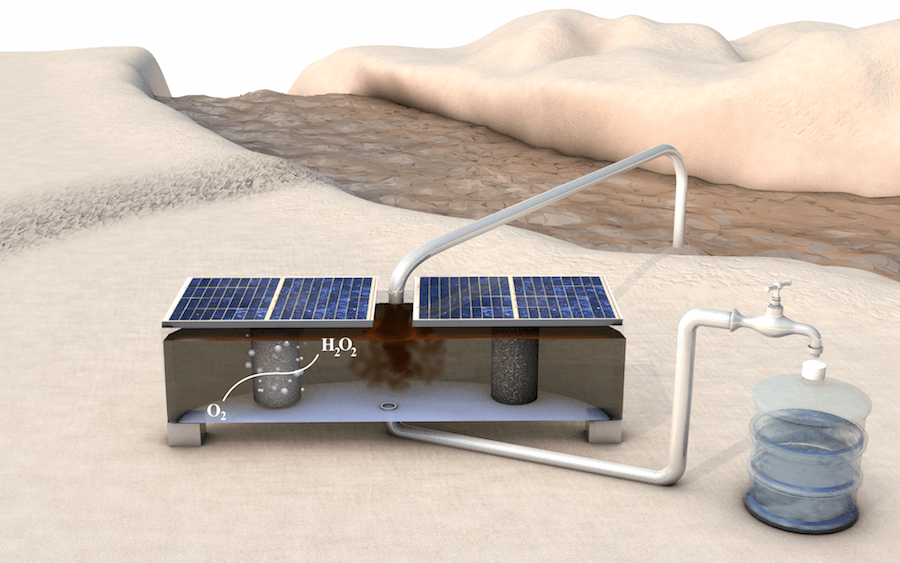 Schematic illustration of an on-site water purification system for rural communities. Powered by solar panels, the low-cost, portable device produces hydrogen peroxide from oxygen gas and water. (Zhihua Chen/Stanford University)
Schematic illustration of an on-site water purification system for rural communities. Powered by solar panels, the low-cost, portable device produces hydrogen peroxide from oxygen gas and water. (Zhihua Chen/Stanford University)
Water sources are usually contaminated with industrial, urban, and agricultural waste in the developing world, and hence, limited access to clean water is a major issue for billions of people. Hydrogen peroxide can be employed to quickly remove many disease-causing organisms and organic pollutants, with no trace of any harmful residual chemicals as remnants. However, it is challenging to produce and distribute hydrogen peroxide in several parts of the world.
Now, a small device has been developed by scientists at the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University for producing hydrogen peroxide. This is driven by renewable energy sources, similar to the traditional solar panels.
The idea is to develop an electrochemical cell that generates hydrogen peroxide from oxygen and water on site, and then use that hydrogen peroxide in groundwater to oxidize organic contaminants that are harmful for humans to ingest.
Chris Hahn, Associate Staff Scientist, SLAC
The results of their research were reported in the March 1 issue of the Reaction Chemistry and Engineering.
This project was a collaborative effort involving three research groups at the SUNCAT Center for Interface Science and Catalysis. The SUNCAT Center is jointly operated by Stanford University and the SLAC.
“Most of the projects here at SUNCAT follow a similar path,” said Zhihua (Bill) Chen, a graduate student in the group of Tom Jaramillo, an associate professor at SLAC and Stanford. “They start from predictions based on theory, move to catalyst development, and eventually produce a prototype device with a practical application.”
Teamwork
The theory group researchers, headed by SLAC/Stanford Professor Jens Nørskov, employed atomic scale-computational modeling to study carbon-based catalysts, which can increase the efficiency and reduce the cost of hydrogen peroxide production. On investigation, the group found that most defects in these materials are naturally selective for producing hydrogen peroxide, and some are also extremely active. During the growth process, defects can be naturally formed in the carbon-based materials. Hence, the important finding was to develop a material with a large number of defects.
My previous catalyst for this reaction used platinum, which is too expensive for decentralized water purification. The beautiful thing about our cheaper carbon-based material is that it has a huge number of defects that are active sites for catalyzing hydrogen peroxide production.
Samira Siahrostami, Research Engineer, SLAC
Then, Stanford Professor Zhenan Bao’s graduate student Shucheng Chen developed the carbon catalysts and determined their properties. SSRL staff scientists Dimosthenis Sokaras and Dennis Nordlund employed X-rays at the SLAC’s Stanford Synchrotron Radiation Lightsource (SSRL) to characterize these catalysts. SSRL is a DOE Office of Science User Facility.
“We depended on our experiments at SSRL to better understand our material’s structure and check that it had the right kinds of defects,” Shucheng Chen said.
Finally, Shucheng Chen’s roommate, Bill Chen, used the catalysts to design, build and test the device.
“Our device has three compartments,” Bill Chen explained. “In the first chamber, oxygen gas flows through the chamber, interfaces with the catalyst made by Shucheng and is reduced into hydrogen peroxide. The hydrogen peroxide then enters the middle chamber, where it is stored in a solution.” Another catalyst is employed to convert water into oxygen gas in the third chamber, and the cycle starts over.
It is simpler, less expensive and more robust to separate the two catalysts in a middle chamber as compared to their separation using a standard semi-permeable membrane, which is susceptible to be attacked and degraded by the hydrogen peroxide.
Villages have renewable energy sources, which can also be employed to run the device. The electrochemical cell is actually an electrical circuit, which is operated by applying a small voltage applied across it. The reaction in the first chamber puts electrons into oxygen to produce hydrogen peroxide, which is balanced by a counter reaction in the third chamber, which makes oxygen by taking electrons from water — matching the current and completing the circuit. The device needs about 1.7 V to be applied between the catalysts, and thus, can run on two standard solar panels or a battery.
The Future
Presently, the research groups are working to develop a higher-capacity device.
The groups wish to increase the capacity of the middle chamber which now holds only about 10 microliters of hydrogen peroxide; they want to make it bigger. The group is also trying to make the liquid circulate in the middle chamber in a continuous manner in order to rapidly pump hydrogen peroxide out, thus avoiding the storage chamber’s size limiting the production.
Higher concentrations of hydrogen peroxide are also a requirement. However, for treating one liter of water, only a few milligrams of hydrogen peroxide are needed. Hydrogen peroxide is produced in sufficient concentration by the current prototype, which is one-tenth of the concentration of the hydrogen peroxide that can be bought at the store for basic medical requirements.
Moreover, considering the long term requirements, the team wishes to change the alkaline environment inside the cell to a neutral one, something similar to water. This would facilitate easier usage, as hydrogen peroxide could be directly mixed with drinking water, thus avoiding the process of neutralization.
Encouraged with the results, the team members believe that they are on the correct path to develop a practical device.
Currently it’s just a prototype, but I personally think it will shine in the area of decentralized water purification for the developing world. It’s like a magic box. I hope it can become a reality.
Zhihua (Bill) Chen, Graduate Student, Stanford University
Pongkarn Chakthranont was also a part of the research team at the SUNCAT Center for Interface Science and Catalysis. The Stanford Dean of Engineering supported the work, which drew on foundational research in catalysis supported by the DOE Office of Science.