Apr 27 2017
 Copyright M.Gratzel/EPFL
Copyright M.Gratzel/EPFL
Perovskites are materials that have currently gained immense interest in solar energy technology as they prove to reduce the cost of solar cells to extremely low levels. Their efficiency majorly depends on the ability of individuals to control their deposition into films, however, the factors that influence the synthesis of perovskites are yet to be fully understood.
EPFL scientists have recently proved that in the two most common methods used today, light can immensely accelerate the formation of perovskite films and also control the morphology of their crystals, thus influencing the competence of the resulting solar cell. The breakthrough study features in Nature.
The research was performed by the lab of Michael Grätzel at EPFL. Grätzel has been globally recognized for inventing the first dye-sensitized solar cells (Grätzel cells) that modernized solar-energy science.
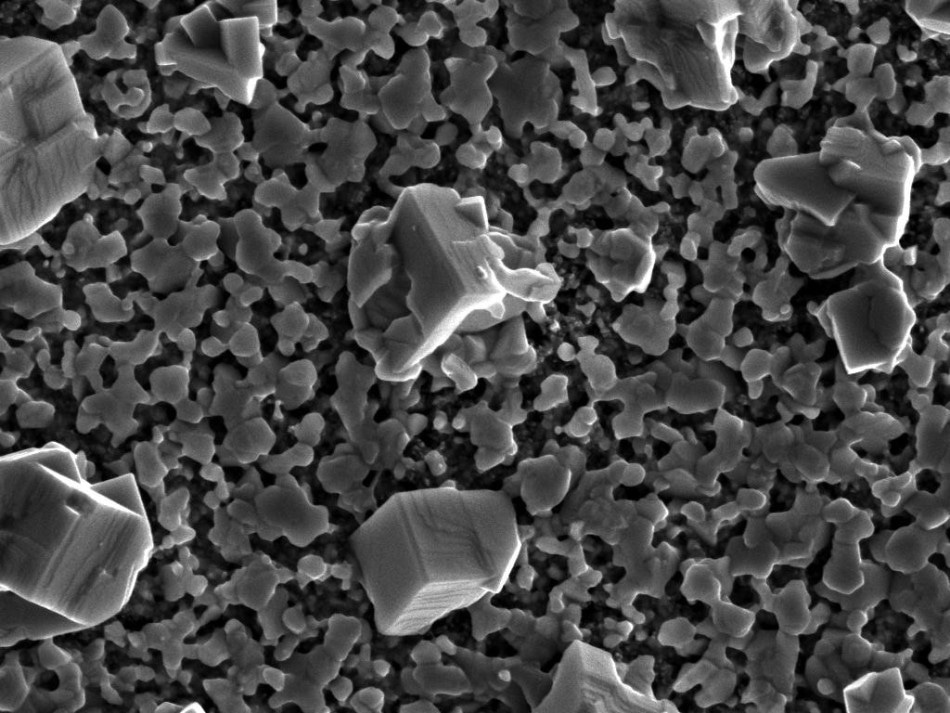
In this paper, Amita Ummadisingu, a PhD student, and her colleagues at Grätzel’s lab used scanning electron microscopy and confocal laser scanning fluorescence microscopy to study how direct light affects the crystal formation when depositing perovskites in layers, as it generally done to build a solar cell. The aim here is to guarantee homogeneity across the perovskite film, as this is connected to superior photovoltaic performance
Till date, efforts have been focused on enhancing perovskite solar-cell efficiency by optimizing the fabrication processes and their architecture. But basic aspects of perovskite formation, such as the factors or mechanism controlling the reaction are not properly understood, resulting in batch-to-batch inconsistencies in performance.
Light can improve the efficiency of perovskite solar cells
It should indeed be noted that understanding the light effect can enable scaling-up and reproducibility of perovskite photovoltaics to commercial production. This resulted in EPFL scientists studying the effect of light on two methods that are commonly used today for perovskite film deposition.
Sequential deposition, the first method, involves depositing lead iodide onto a titanium dioxide scaffold. This is followed by dipping them together in a methylammonium iodide solution in order to produce the final methylammonium lead iodide perovskite. The anti-solvent method is the second commonly used method in which a perovskite precursor solution is uniformly coated onto a spinning flat base to produce a thin layer. This is then followed by dripping an anti-solvent and the whole sample is finally heated to produce the perovskite.
The scientists used both the microscopy techniques during the sequential deposition method and discovered that when light is present, it significantly speeds up the overall formation of the perovskite. The effect is improved when light intensity is increased to solar levels.
The morphological differences observed in the film are not because of heating from the light but due to the product of a quantum effect: the lead iodide film crystallizes as the first step in perovskite formation, and then develops surface traps. Photo-generated holes, reducing the surface tension, are captured by these traps. This results in the growth of more lead iodide crystals in the film, ultimately developing more perovskite crystals.
The researchers also discovered that the anti-solvent method actually gains a lot more from the absence of light, and indeed provides best results in the dark.
The study highlights that controlling the light present during fabrication can help attain superior performance from perovskite solar cells. “Surprisingly and strikingly we find that light strongly affects the rate of formation and the morphology of perovskites in conventional methods used where no light effects have been remarked before,” says Michael Grätzel.
Light is a major factor in the main deposition methods and for many perovskite compositions. So awareness and control of the light effect is crucial when fabricating state-of-the-art perovskite devices, and should always be considered when preparing perovskite films for opto-electronic applications such as solar cells, LEDs, etc.
Amita Ummadisingu, PhD Student, EPFL
The Swiss National Science Foundation (SNSF) funded this work.