May 4 2017
At present, lithium ion batteries occupy a prominent place in the rechargeable battery industry. In the recent times, sodium ion batteries have been gaining more attention as a substitute to lithium ion batteries as sodium is naturally more abundant when compared with lithium.
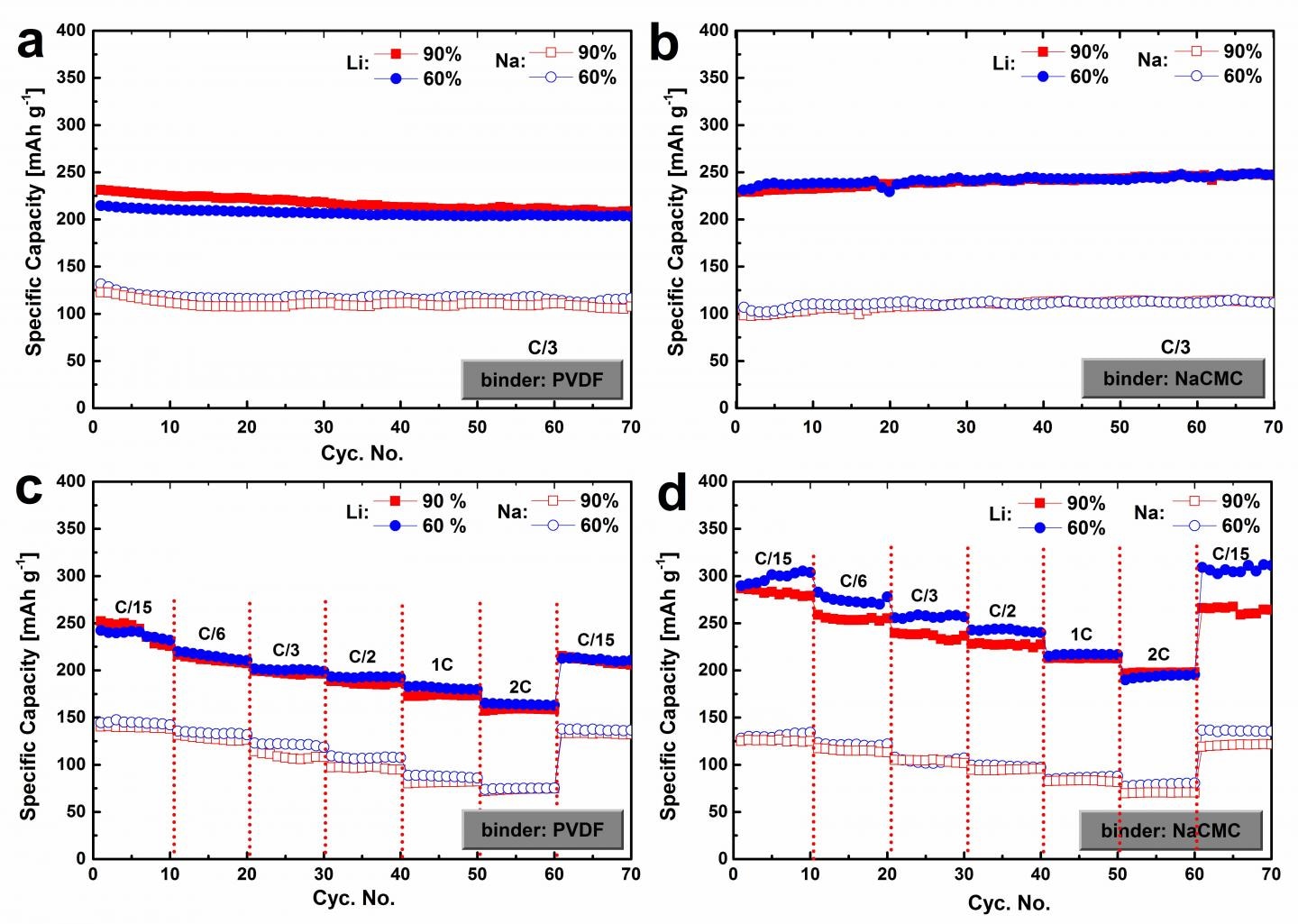 This image shows electrochemical lithium/sodium ion storage performance of the SPCB based electrodes containing 90 percent and 60 percent of active materials (C = 300 mA g-1). CREDIT: Science China Press.
This image shows electrochemical lithium/sodium ion storage performance of the SPCB based electrodes containing 90 percent and 60 percent of active materials (C = 300 mA g-1). CREDIT: Science China Press.
Super P carbon black (SPCB)—synthesized by partial oxidation of petrochemical precursors that have exceptional electrical conductivity and a large specific surface area—is the conducting additive generally used in the lithium/sodium ion battery electrodes for enhancing the electronic conductivity. Yet, to date, there is only restricted understanding of the structure, reaction mechanisms, and electrochemical characteristics for sodium/lithium ion uptake.
An elaborate research on the electrochemical properties and structure of SPCB has been described in an article titled “The electrochemical performance of super p carbon black in reversible Li/Na ion uptake,” which has been published in the Science China Physics, Mechanics & Astronomy journal. The article has been coauthored by scientists from the Delft University of Technology, Renmin University of China and the National Center for Nanoscience and Technology of China.
In order to unearth the morphology and structure of SPCB, the research team employed Raman spectroscopy, scanning electron microscopy (SEM), as well as X-ray diffraction (XRD). Consequently, the SPCB was found to be formed of flocculent, porous, and cross-linked carbon nanothreads. The SPCB is generally amorphous and includes considerable structural disorder, that is, low graphitization level.
The electrochemical performance of SPCB denotes that SPCB has higher cycling stability, substantial capacity for storing sodium as well as lithium ions, and exceptional rate capability. It has been shown that SPCB attains a higher capacity for the storage of lithium when compared to that for sodium ion. A reversible capacity of about 310 mAh g-1 was recorded for lithium ion uptake. For the sodium ion uptake, it was 145 mAh g-1. The researchers attribute this to the disparate reaction mechanisms between sodiation and lithiation of SPCB. Graphite intercalation compounds (GICs) are formed by the intercalation of the lithium ions in the graphite structure of SPCB. However, this does not hold good for sodium ion insertion. The article specifies that the low graphitization level of SPCB causes the sodium ion uptake in SPCB to follow the “card-house” model where there are two stages: intercalation of sodium ions in the layers between the sheets of graphene, and sodium plating in the pores in-between the nano-graphitic domains.
For the first time, the research has also explored the impact of binder content and type on the electrochemical sodiation and lithiation performance of SPCB. Electrodes made using the water-soluble NaCMC binder exhibit excellent cycling stability when compared with the electrodes made of PVDF—the mainstream binder in commercial products and battery research. “This can be probably attributed to not only the superior adhesive quality and elasticity of NaCMC but the formation of a more compact SEI layer upon the presence of FEC in the electrolyte,” explained the research, thus assisting in the maintenance of the structural integrity during cycling, and hence accomplishing higher cycling stability. In the mean time, the article shows that when the binder content in the electrode is increased, there is an increase in the Coulombic efficiency. The increase in the amount of binder also results in enhanced retained structural integrity during cycling.
The extraction and insertion of lithium ion in SPCB predominantly takes place at a low voltage range, indicating a sloping voltage that is less than 1.2 V. However, the desodiation/sodiation occurs at an even lower voltage of less than 0.8 V. The sodiation voltage profile involves a comparatively high voltage slope and a voltage plateau close to 0 versus Na/Na+, which is compatible with the insertion of Na ion based on the “card-house” model. “The consequence of the sloping voltage profiles,” according to the researchers, “will be that in a low potential lithium/sodium ion anode (e.g. Li-Si or Na-P using SPCB as a conducting additive) the SPCB will largely be lithiated or sodiated at a higher potential than where the main lithiation or sodiation of the active materials takes place. For higher potential anodes (such as Li4Ti5O12) or cathodes no lithiation or sodiation of SPCB will take place.”
Reporting on the regular use of SPCB in battery analyses, the research team explained that “The electrochemical lithium/sodium ion uptake properties of SPCB reported in this article will provide an essential reference for the research on lithium and sodium ion battery electrodes that utilize significant amounts of SPCB as a conductive additive.”