Aug 29 2017
Metal oxides are highly favorable for producing stable and inexpensive photoelectrodes for solar water splitting by using sunlight to produce hydrogen. However, metal oxides are not so efficient in carrying out the process.
A treatment with hydrogen and heat help overcome this. An international research team has found out why this treatment works well, thus opening the door for producing inexpensive and highly efficient devices for producing hydrogen using solar energy.
 CREDIT: HZB.
CREDIT: HZB.
The era of fossil fuels is nearing an end, for many reasons. Hydrogen looks highly attractive as a substitute to fossil fuels as it has high energy density, that can be stored or processed into fuels such as methane, or can directly supply clean electricity when used in a fuel cell. When hydrogen in produced by using only sunlight, it is completely renewable with zero carbon emissions.
Artificial leafs
Simliar to the process that takes place during natural photosynthesis, “artificial leafs” can be used to tap sunlight to split water into hydrogen and oxygen. The artificial leafs are made of photoactive semiconducting materials and can achieve efficiencies of more than 15%. Yet, such record efficiencies were achieved by means of costly systems that are known to be decomposed when placed in aqueous solutions. If this process has to be successfully commercialized, the costs have to be reduced and stability enhanced.
Good candidates with one disadvantage
The use of complex metal oxide semiconductors for making artificial leafs seems to be highly promising as these materials are cheap and highly stable when placed in aqueous solutions. The focus of researchers at the HZB-Institute for Solar Fuels is on these materials. Till date, metal-oxide-based photoelectrodes have exhibited only moderate efficiencies of less than 8% mainly due to poor mobility of their charge carriers (electron and/or hole), where the mobility is nearly 100,000 times when compared to that of classical semiconductors (e.g. silicon or gallium arsenide).
What is worse is the fact that charge carriers in metal oxides often have really short life spans of nanoseconds or even picoseconds. Many of them disappear before they can contribute to water splitting.
Dr. Fatwa Abdi, expert at HZB-Institute for Solar Fuels.
Heat treatment with hydrogen
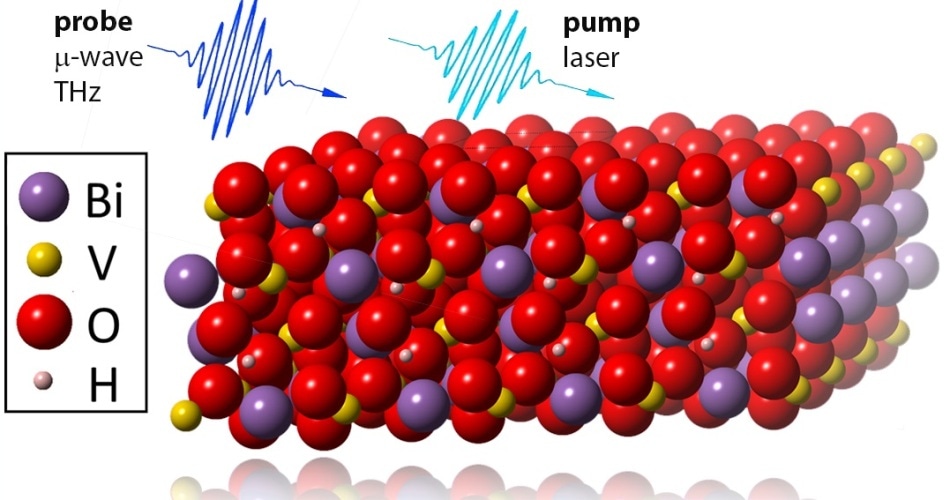
One way of fixing this issue is to carry out heat treatment of the metal oxide layers after deposition under hydrogen atmosphere. At present, Fatwa Abdi and his team have analyzed the way this treatment has an impact on transport characteristics, service life, and flaws in one of the highly propitious metal oxide photoelectrodes, namely, bismuth vanadate, or BiVO4.
Life spans of charge carriers doubled
When time-resolved conductivity measurements were carried out on BiVO4, it could be inferred that the life span of holes and electrons was nearly twice as that in the hydrogen-treated BiVO4 as against the pristine BiVO4. Consequently, the average photocurrent synthesized from sunlight is greatly increased. Additional measurements carried out at Dresden and theoretical computations performed by collaborators from KAUST in Saudi Arabia presented evidence to confirm that the existence of hydrogen in the metal oxide decreases or deactivates point defects in the bulk of BiVO4.
Our results show that hydrogen treatment leads to less traps for charge carriers and less opportunities to recombine or getting lost. So more charge carriers survive for longer and may contribute to water splitting.
Abdi.