Sep 8 2017
New techniques for making 3D-printed biomaterials capable of degrading on demand have been developed by Brown University Engineers. This technology can be productive in making cell cultures than can vary dynamically during experiments or in making intricately patterned microfluidic devices.
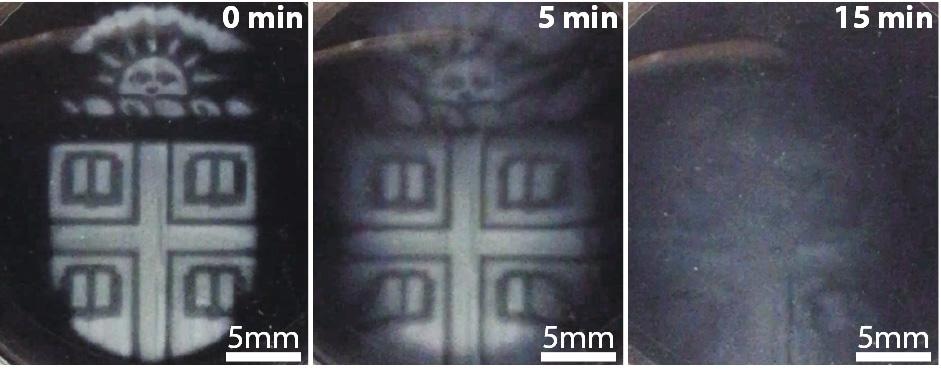 Brown researchers have found a way to 3-D print intricate temporary microstructures that can be degraded on demand using a biocompatible chemical trigger. (Wong Lab / Brown Univeristy)
Brown researchers have found a way to 3-D print intricate temporary microstructures that can be degraded on demand using a biocompatible chemical trigger. (Wong Lab / Brown Univeristy)
It’s a bit like Legos. We can attach polymers together to build 3-D structures, and then gently detach them again under biocompatible conditions.
Ian Wong, Assistant Professor, Brown’s School of Engineering and Co-author of the research
This study has been published in the journal Lab on a Chip.
The Brown team created their innovative degradable structures using a type of 3D printing known as stereolithography. In order to trace patterns across the surface of a photoactive polymer solution, this technique uses an ultraviolet laser controlled by a computer-aided design system. The light causes the polymers to connect together, producing solid 3D structures from the solution. The tracing process is carried out continuously until a complete object is developed from the bottom up.
Usually, stereolithographic printing uses photoactive polymers that connect together with covalent bonds, which are irreversible but strong. For this latest research, Wong and his colleagues desired to try creating structures with potentially reversible ionic bonds, which had never been performed earlier employing light-based 3D printing. To do it, the Researchers prepared precursor solutions with sodium alginate, a compound derived from seaweed that is well acknowledged to be capable of ionic crosslinking.
The idea is that the attachments between polymers should come apart when the ions are removed, which we can do by adding a chelating agent that grabs all the ions. This way we can pattern transient structures that dissolve away when we want them to.
Ian Wong, Assistant Professor, Brown’s School of Engineering and Co-author of the research
The Researchers proved that alginate could certainly be used in stereolithography. Additionally, when employing different combinations of ionic salts — calcium, barium and magnesium — structures with altering stiffness could be created, which could then be dissolved away at different rates.
The research also demonstrated multiple ways in which such temporary alginate structures could be productive.
“It’s a helpful tool for fabrication,” said Thomas M. Valentin, a Ph.D. Student in Wong’s lab at Brown and the Lead Author of the research. The Researchers demonstrated that an alginate could be used as a template for manufacturing lab-on-a-chip devices with intricate microfluidic channels.
We can print the shape of the channel using alginate, then print a permanent structure around it using a second biomaterial. Then we simply dissolve away the alginate and we have a hollow channel. We don’t have to do any cutting or complex assembly.
Thomas M. Valentin, a Ph.D. Student in Wong’s lab at Brown and the Lead Author of the research
In addition, the Researchers demonstrated that degradable alginate structures are productive for creating dynamic environments for experiments with live cells. They carried out a series of experiments with alginate barriers bounded by human mammary cells, and observed the pattern of cell migration when the barrier is dissolved away. Such experiments can be productive in studying the migration of cells or wound-healing processes in cancer.
From the experiments, it is clear that neither the chelating agent nor the alginate barrier used to dissolve it away had any significant toxicity to the cells. This reveals that degradable alginate barriers are a prospective option for such experiments.
The Researchers say that the biocompatibility of the alginate shows potential for further future applications, including in developing scaffolds for artificial organs and tissues.
“We can start to think about using this in artificial tissues where you might want channels running through it that mimic blood vessels,” Wong said. “We could potentially template that vasculature using alginate and then dissolve it away like we did for the microfluidic channels.”
The Researchers plan to carry on experimenting with their alginate structures, in search of ways to fine-tune not only the pace of degradation but also their stiffness and strength properties.
Besides Valentin and Wong, Co-authors on the paper were Susan Leggett, Po-Yen Chen, Jaskiranjeet Sodhi, Lauren Stephens, Hayley McClintock and Jea Yun Sim. The Department of Education (P200A150037), National Institutes of Health (5T32ES007272-24), Brown’s Hibbitt Postdoctoral Fellowship and the Center for Cancer Research and Development at Rhode Island Hospital (1P30GM110759-O1A1) supported this research.