Sep 15 2017
The National Institute of Standards and Technology (NIST) researchers have developed a laboratory instrument capable of measuring the amount of carbon in many carbon-containing materials derived from fossil fuels.
This will allow for the development of new methods in the bioplastics and biofuels industries, in environmental monitoring, and scientific research. Among various other things, it will allow researchers to measure how much of the carbon dioxide (CO2) present in the atmosphere is coming from burning fossil fuels, and calculate fossil fuel emissions in an area as large as a continent and as small as a city.
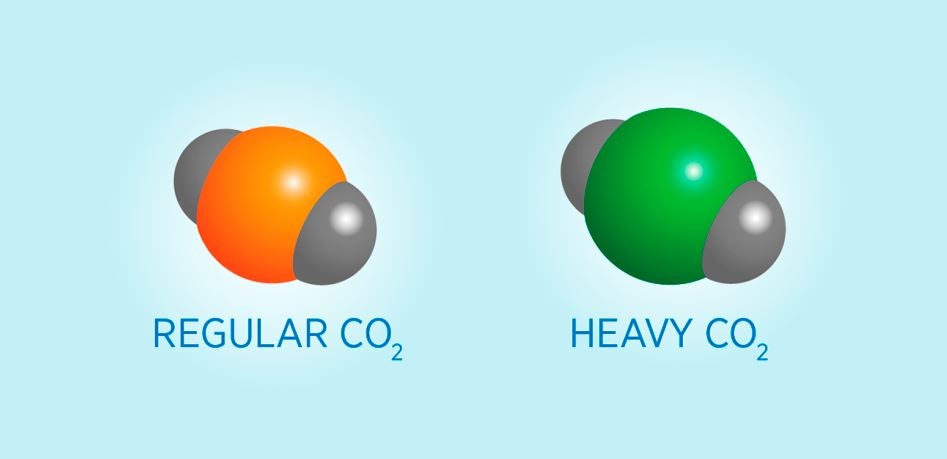 Carbon atoms occur in heavy and light forms, or isotopes, and measuring the relative amounts of each can reveal the source of the carbon. Oxygen atoms are represented in gray and carbon isotopes are in orange and green. Credit: Kelly Irvine/NIST
Carbon atoms occur in heavy and light forms, or isotopes, and measuring the relative amounts of each can reveal the source of the carbon. Oxygen atoms are represented in gray and carbon isotopes are in orange and green. Credit: Kelly Irvine/NIST
Since carbon atoms occur in light and heavy forms, this measurement is possible, and measuring the proportional amounts of each can reveal the carbon source. It is not a new idea to use carbon isotopes in this way; however, it requires very precise and expensive measurements. Developed by NIST chemists David Long and Adam Fleisher and based on a technology known as cavity ringdown spectroscopy (CRDS), the new instrument promises to reduce the cost of those measurements significantly. The researchers described the performance of the new instrument in The Journal of Physical Chemistry Letters.
Measuring carbon isotopes is an extremely useful technique, but until now, it has found limited use because of the cost. Lowering the cost will open the way for new applications, especially ones that require testing a large number of samples.
Long.
The solution to these measurements is carbon-14, a radioactive (but harmless) isotope of carbon that is produced in the upper atmosphere. This carbon-14 finds its way into every living thing. Unlike standard carbon, carbon-14 is unstable and has a half-life of 5,730 years. After living things die, they stop absorbing carbon into their bodies, and their carbon-14 begins to decay away.
Researchers can determine how long ago something died by calculating how much carbon-14 is in its remains. This process is known as carbon dating, and researchers use it to date things such as Neanderthal bones and ancient plant fibers.
Fossil fuels are also the remains of living things, primarily plants that died hundreds of millions of years ago. Almost all their carbon-14 decayed away eons ago, therefore, anything originated from them is marked the absence of calculable amounts of carbon-14.
However, carbon-14 is very rare, and researchers have to measure it at concentrations as low as 1 part in 10 trillion in order to use it for identifying fossil fuels. This is equivalent to a single grain of sand in 60 dump trucks full of the stuff.
In order to measure low concentrations, researchers require a very sensitive measurement technique, and this type of technique is already available and archaeologists have been using it for decades. However, this technique needs a particle accelerator in order to separate the isotopes (the heavier carbon-14 speeds up more slowly than everyday carbon-12), together with a facility to accommodate it and a team of PhDs to manage it.
The CRDS instrument, developed by Fleisher and Long, can be put on a laboratory benchtop and is comparatively inexpensive to operate.
CRDS instruments examine gases by detecting the light wavelengths they absorb. For example, CO2 that contains carbon-14, so-called heavy CO2, absorbs a slightly different wavelength compared to regular CO2. In order to measure how much heavy CO2 the users have in a CO2 sample, first they need to inject the sample into the measurement cavity (the “C” in CRDS) of the instrument, where the measurement cavity is a tube with mirrors inside at each end. Then, the users have to tune a laser to the precise wavelength that only heavy CO2 absorbs and release a burst of it into the cavity. When the laser light bounces between the mirrors, the gas absorbs some of the energy of the laser light. When the absorption is greater, the concentration of heavy CO2 will also be greater.
In order to obtain the required sensitivity, Fleisher and Long improved existing CRDS technology by producing a system that chills the cavity to a uniform - 55 °C and reduces temperature fluctuations that may throw off the measurement. Making the cavity extremely cold enables their instrument to detect very faint light absorption signals, the same way that they might be able to hear a pin drop if they made a room very quiet.
This and other improvements enhanced the instrument’s sensitivity for accurate carbon dating. In order to test bioplastics and biofuels, users must first burn those materials, and then gather the resulting CO2 for analysis. This would enable them to test a fuel mixture in order to determine what fraction of it is biofuel. For instance, in the airline industry, this would be beneficial because some countries need that aviation fuels a specific biofuel percentage. These types of tests could also be employed to confirm that bioplastics, which sell for a premium, do not include petroleum-derived compounds.
In order to estimate fossil fuel emissions in a particular geographic area, researchers would need to collect many air samples across that area and examine the atmospheric CO2 in those samples. In those areas with high fossil fuel emissions, such as industrial zones and cities, the concentrations of heavy CO2 will be below normal.
Fossil fuel emissions dilute the concentration of heavy CO2 in the air. If we can accurately measure that concentration after it’s been diluted, we can calculate how much fossil fuel emissions are in the mix.
Fleisher
The National Academy of Sciences’ report estimated that 10,000 samples a year, collected from different locations around the United States, would be sufficient to estimate national fossil fuel emissions to within 10% of the actual value. Such a system of measurements can boost the reliability of national emissions estimates. This would be particularly useful in areas where high-quality emissions data are not easily available.
There is a need for this type of measurement in many industries. We’ve demonstrated a path to meeting that need in a cost-effective way.
Fleisher