Feb 19 2018
According to researchers from the Osaka City University in Japan, eggs may soon be used to fuel more than just people in the morning. These researchers have formulated a way to potentially use egg whites as a substrate to create a carbon-free fuel.
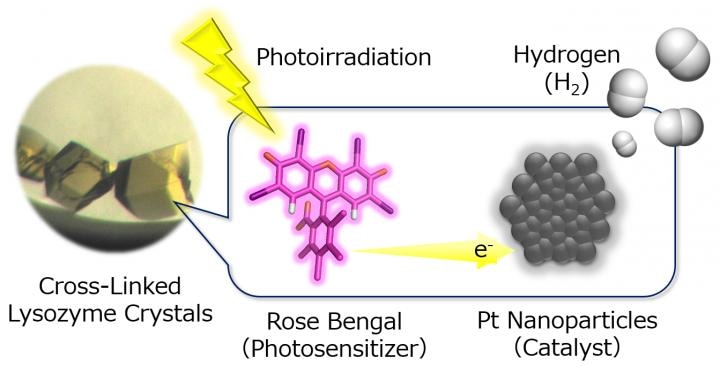 Hydrogen (H2) evolution systems constructed in cross-linked porous lysozyme crystals by immobilizing Pt nanoparticles as H2-evolution catalysts in immediate proximity to an organic photosensitizer, rose bengal. (Image credit: H. TABE/Osaka City University)
Hydrogen (H2) evolution systems constructed in cross-linked porous lysozyme crystals by immobilizing Pt nanoparticles as H2-evolution catalysts in immediate proximity to an organic photosensitizer, rose bengal. (Image credit: H. TABE/Osaka City University)
They published their findings on February 2, 2018, in Applied Catalysis B.
Hydrogen is a promising fuel and energy storage medium because hydrogen emits no global warming gas when used. Nevertheless, hydrogen generation reactions usually require fossil fuels and emit carbon dioxide.
Hiroyasu Tabe, Special Appointment Research Associate - Graduate School of Engineering at Osaka City University, Japan.
According to Tabe, it would be very efficient to use a photocatalyst to accelerate the reaction of hydrogen generation from a renewable source, such as solar power. Referred to as hydrogen evolution, the gas must be stored and kept from recombining into more common molecules that aren’t beneficial for making clean fuel.
“Precise accumulation of molecules acting as catalytic components are important to construct a photocatalytic system,” Tabe said. “When the molecular components are randomly distributed in the solution or formless compounds, the catalytic reactions cannot proceed.”
One favorable way to precisely collect these catalytic molecules is through the creation of pure proteins by cultivated bacteria, but they necessitate special lab equipment. Chicken eggs, however, are well-known receptacles of protein-based chemicals, according to Tabe.
The whites of chicken eggs, which are inexhaustible and inexpensive, consist of porous lysozyme crystals.
“Lysozyme crystals have a highly ordered nanostructure and, thus, we can manipulate the molecular components when they accumulate in the crystals,” Tabe said, noting that the crystal structure can be easily examined with X-ray technology.
This analysis is of particular significance, according to Tabe, as the molecular components within the crystals must be exploited precisely through what is termed as cooperative immobilization. This is accomplished by the application of rose bengal, which is frequently used as a dye in eye drops to detect damage. In this case, it entered the solvent channels in the lysozyme crystals and hastened the hydrogen evolution reaction, since the functional molecules and nanoparticles can be collected within the crystals’ inner spaces.
“These results suggest that porous protein crystals are promising platforms to periodically and rationally accumulate catalytic components by using molecular interactions,” Tabe said.