Mar 9 2018
In association with scientists from Institute of Chemical Research in Catalonia, Spain, ITQB NOVA chemists from Beatriz Royo Lab have developed a highly selective catalyst that can reduce carbon dioxide to carbon monoxide. Carbon dioxide is a greenhouse gas, while carbon monoxide can be used for developing value-added chemicals.
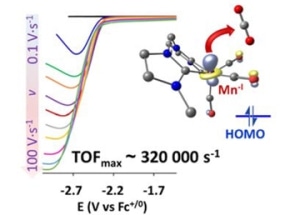 Image credit: ITQB NOVA
Image credit: ITQB NOVA
The results of the study have been reported in Angew. Chem. Int. Ed. Angewandte Chemie, a prominent chemistry journal with Impact Factor of 11.994.
A significant challenge in sustainable chemical research is the large-scale production of useful fuels and chemicals using carbon dioxide and renewable energy as feedstock. In this regard, electrochemical reduction of carbon dioxide has always presented an interesting means to transform atmospheric carbon dioxide into commodity products.
In this study, the scientists have developed a new range of manganese complexes bearing N-heterocyclic carbenes that can perform electrocatalytic reduction of carbon dioxide to carbon monoxide with incredible activity and productivity, surpassing the values reported to date for other catalysts based on manganese. An interesting fact is that the active species operating in this process were detected by these manganese complexes.
We have synthesized the first pure organometallic manganese complexes for the activation of CO2, and we were very excited to find out that they display unprecedented catalytic activity for converting carbon dioxide to carbon monoxide. The electrochemical reduction of carbon dioxide to CO is a major step to the utilization of CO2 as a cheap and renewable source of carbon.
Beatriz Royo, Group Leader - Organometallic Catalysis Lab