May 1 2018
At present, two of the most significant challenges common to developed countries are taking steps to address climate change and translating into communities that use considerable amounts of renewable energy for power.
One propitious technology for achieving these involves the use of hydrogen (H2) as a source of renewable energy. Despite the fact that hydrogen is a basic candidate for clean secondary energy, huge amounts of H2 must be transformed into liquid form, which is a challenging procedure, for easier storage and transportation. Of the probable forms of liquid H2, ammonia (NH3) is a propitious carrier since it has a high density of H2, can be easily liquefied, and can be synthesized on a large scale.
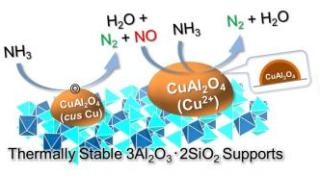 CuOx/3A2S selectively produces N2 and H2O from NH3 through a two-step reaction. (Image credit: Dr Satoshi Hinokuma)
CuOx/3A2S selectively produces N2 and H2O from NH3 through a two-step reaction. (Image credit: Dr Satoshi Hinokuma)
Moreover, recently, NH3 has been gaining attention as a carbon-free substitutive fuel. NH3 is a combustible gas that can be largely applied in industrial furnaces and thermal power generation as a substitute for light oil and gasoline. However, it is hard to ignite (due to its high ignition temperature) and produces hazardous nitrogen oxides (NOx) at the time of combustion.
The focus of scientists from the International Research Organization for Advanced Science and Technology (IROAST) in Kumamoto University, Japan, was on a “catalytic combustion method” to solve the NH3 fuel challenges. This technique adds substances that suppress or promote chemical reactions at the time of fuel combustion.
In the recent past, the researchers were successful in creating an innovative catalyst that suppresses the production of NOx and enhances NH3 combustibility. The innovative catalyst (CuOx/3A2S) is a mullite-type crystal structure 3Al2O3·2SiO2 (3A2S) carrying copper oxide (CuOx). When this catalyst was used to burn NH3, scientists discovered that it remained highly active in the selective synthesis of N2, implying that it suppressed the formation of NOx, and the catalyst did not change itself even at higher temperatures. Furthermore, they were successful in their in situ (Operando) observations during the CuOx/3A2S reaction and refined the NH3 catalytic combustion reaction mechanism.
Due to the fact that 3A2S is a commercially available material and CuOx can be synthesized with the help of a technique largely used in industry (wet impregnation technique), this innovative catalyst is inexpensive and easy to produce. The use of this catalyst enables the disintegration of NH3 into H2 with the heat from (low ignition temperature) NH3 fuel combustion, and the purification of NH3 by means of oxidation.
Our catalyst appears to be a step in the right direction to fight anthropogenic climate change since it does not emit greenhouse gasses like CO2 and should improve the sophistication of renewable energy within our society. We are planning to conduct further research and development under more practical conditions in the future.
Dr. Satoshi Hinokuma of IROAST, Study Leader
This study was published online on March 26, 2018, in the Journal of Catalysis.