May 17 2018
Xiao Cheng Zeng and his team from the University of Nebraska–Lincoln have identified various neglected factors that are crucial for the performance of single-atom catalysts - individual atoms, normally metallic and anchored by adjacent molecular frameworks, initiating and speeding up chemical reactions.
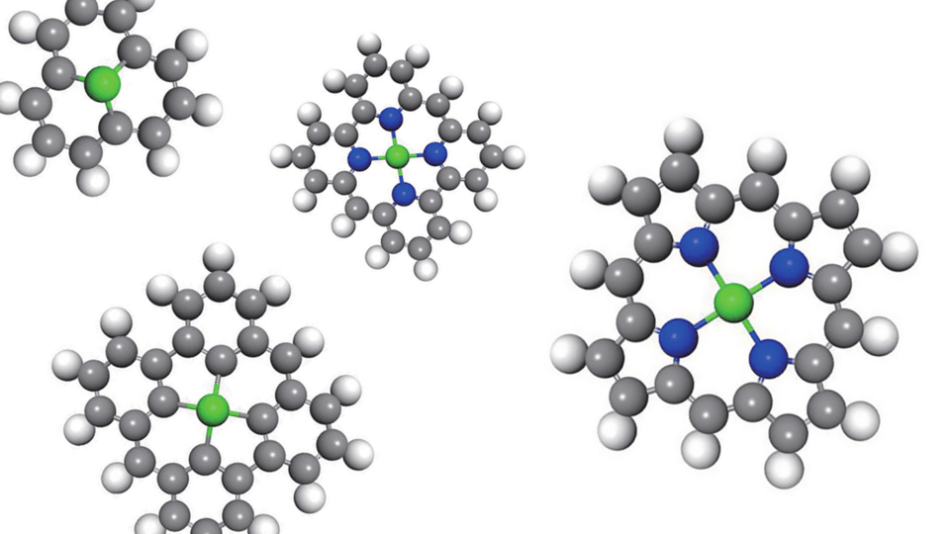 (Image credit: Nature Catalysis/Springer Nature)
(Image credit: Nature Catalysis/Springer Nature)
The researchers integrated those variables into a simple equation that required “back-of-the-envelope calculations,” as described by Zeng. Researchers should be able to use that equation to easily estimate how catalytic performance is affected by the choice of an atom and its surrounding material. So far, scientists have usually relied on trial and error to discover propitious single-atom catalysts, which is time-consuming.
All this (relevant) information can be easily gathered from a textbook. Even before an experiment, you can quickly see whether it’s a good way to make the catalyst. We’re simplifying the process.
Xiao Cheng Zeng, Professor of Chemistry - Chancellor’s University
The team used the equation to find out various atom-framework combinations that simulate the performance of precious-metal catalysts—such as iridium, gold, and platinum—at only one-thousandths of the original cost. In one case, a platinum atom was used in the place of manganese; in another case, iridium was replaced with cobalt.
“There are two (primary) ways to reduce the price of these catalysts,” stated Zeng. “One is to use as little of the metals as possible - so single-atom catalysts are the cheapest. The other direction is finding alternative metals like iron or aluminum or zinc that are very cheap.”
Two of the atom-framework combinations formulated by the researchers have the ability to split water into its constituent parts: two hydrogen atoms and an oxygen atom, where the former can act as a green fuel for vehicles and other applications. Two other catalysts assist oxygen atoms in taking on more electrons, making them to bond with positively charged hydrogen atoms and form water—the sought-after byproduct of hydrogen fuel cells.
Right now, this is not the prevailing way to produce hydrogen,” stated Zeng. “The industry still uses fossil fuels to produce hydrogen. It’s just cheaper. So that’s our motivation: lower the cost so that all these cleaner, fuel-producing reactions become (viable).
Xiao Cheng Zeng, Professor of Chemistry - Chancellor’s University
Scouting Report
The team discovered that the number and nature of atoms that are directly bonded to a single-atom catalyst can have a major impact on the way it catalyzes chemical reactions. In certain cases, the catalyzing atom may be bonded to three or four other atoms, where each atom is part of a five- or six-atom ring. Each atom in that proximate network also has a known attraction to electrons, where the catalytic performance is further influenced by the strength of this attraction.
According to Zeng, the arrangement and qualities of the neighboring atoms have a significant role similar to the role of an offensive line in a stationary, pocket-passing quarterback. Moreover, he also stated that the new equation could serve as a scouting report for researchers seeking to expand the strengths or shield the weaknesses of their candidates.
For Zeng and his team, the candidates included over 20 so-called transition metals that are commonly weak at catalyzing reactions when compared to precious metals. However, the researchers demonstrated that the performance can be increased by surrounding an iron, cobalt, or another second-string atom with the optimal environment - a network of nitrogen atoms, or a honeycomb of carbon atoms called as graphene.
Every offensive line is different,” stated Zeng. “How do you make the quarterback function the best in that pocket? How do you find the best quarterback within different pockets?
If you have a two-star quarterback, you need a better offensive line. But even a backup quarterback can perform well with the right line.
Xiao Cheng Zeng, Professor of Chemistry - Chancellor’s University
The study was authored by Zeng in collaboration with colleagues from Beijing University of Chemical Technology. The research became only the second from a Big Ten institution to have featured in the Nature Catalysis journal and was highlighted in Chemical and Engineering News, a magazine published by the American Chemical Society.