Oct 30 2018
Researchers have successfully harvested solar energy through an artificial photosynthesis system which splits water to generate hydrogen fuel using sunlight.
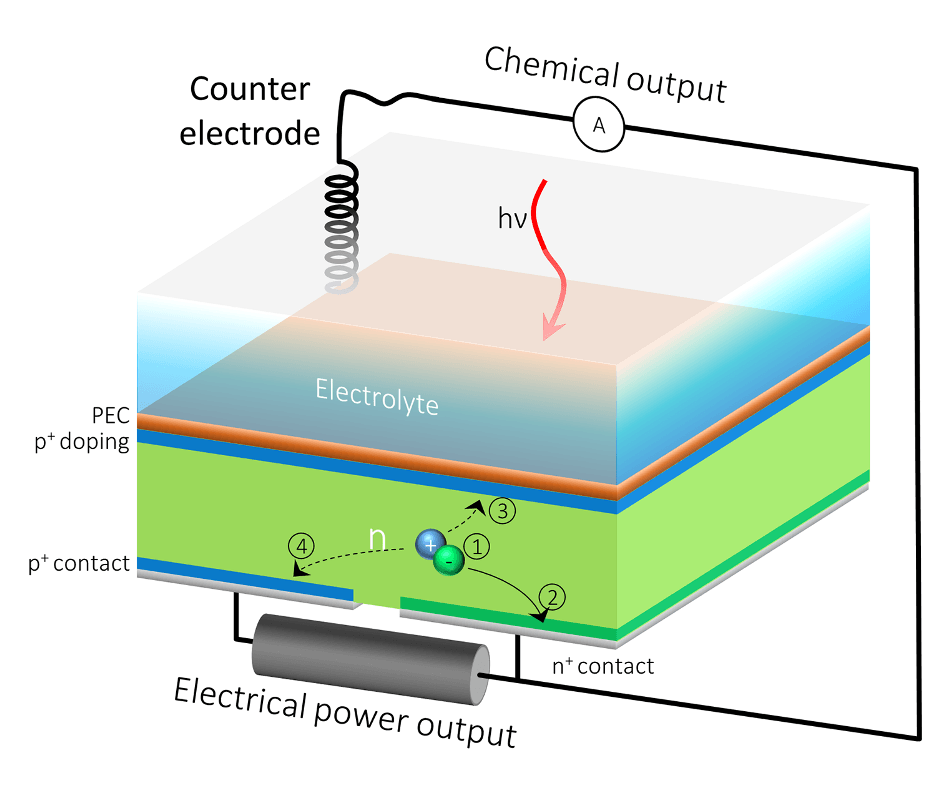 The HPEV cell’s extra back outlet would allow the current to be split into two, so that one part of the current contributes to solar fuels generation, and the rest can be extracted as electrical power. (Credit: Berkeley Lab, JCAP)
The HPEV cell’s extra back outlet would allow the current to be split into two, so that one part of the current contributes to solar fuels generation, and the rest can be extracted as electrical power. (Credit: Berkeley Lab, JCAP)
To date, water-splitting devices have not lived up to their potential because there is still no design available for materials with the right combination of chemical, electronic, and optical properties required for them to function efficiently.
Now, a research team at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) and the Joint Center for Artificial Photosynthesis (JCAP), has developed a novel recipe for renewable fuels that can possibly overcome the restrictions encountered in present-day materials.
The artificial photosynthesis system is known as a “hybrid photoelectrochemical and voltaic (HPEV) cell” and simultaneously converts water and sunlight into two types of energy—electricity and hydrogen fuel—instead of just one. A paper detailing this study recently appeared in Nature Materials.
A stack of light-absorbing materials is used for making a majority of water-splitting devices.
Based on its composition, each layer is able to absorb different “wavelengths” or parts of the solar spectrum, spanning from infrared light’s less-energetic wavelengths to ultraviolet / visible light’s more-energetic wavelengths.
Upon absorbing light, each layer develops an electrical voltage. These separate voltages integrate into a single voltage which is sufficiently large to split water into oxygen as well as hydrogen fuel.
However, Gideon Segev, the study’s lead author and a postdoctoral researcher at JCAP, says that the issue with this type of configuration is that the high-performance potential of silicon solar cells is affected when they are part of a water-splitting device, even though these cells are capable of producing electricity which is very close to their limit.
Other materials in the stack which don’t perform as well as silicon limit the current flowing through the device, and therefore, the system generates relatively less current than it normally would – and when it generates less current, it will produce less solar fuel.
It’s like always running a car in first gear. This is energy that you could harvest, but because silicon isn’t acting at its maximum power point, most of the excited electrons in the silicon have nowhere to go, so they lose their energy before they are utilized to do useful work.”
Gideon Segev, Lead Author
Segev therefore suggested an unexpectedly simple solution to a difficult problem. Working alongside co-authors; Jeffrey W. Beeman from JCAP, Professor Ian Sharp from the Technical University of Munich in Germany, and Jeffery Greenblatt, who currently heads Emerging Futures LLC, he said “We thought, ‘What if we just let the electrons out?".
The front surface of water-splitting devices is often meant for the production of solar fuels, and the back surface acts as an electrical outlet.
In order to work around the limitations of the traditional system, the researchers added an extra electrical contact to the back surface of the silicon component, leading to an HPEV device that has two contacts in the back rather than just one.
With this additional back outlet, the current is allowed to split into two, so that one part of the current plays a role in the generation of solar fuels, while the rest can be removed as electrical power.
The researchers first ran a simulation to predict whether the HPEC would be able to function as expected and then they built a prototype to test their theory. “And to our surprise, it worked!” said Segev.
In science, you’re never really sure if everything’s going to work, even if your computer simulations say they will. But that’s also what makes it fun. It was great to see our experiments validate our simulations’ predictions.”
Gideon Segev, Lead Author
Based on the team’s calculations, a traditional solar hydrogen generator based on a combination of bismuth vanadate—an extensively studied material for solar water splitting—and silicon would produce hydrogen at a solar-to-hydrogen efficiency of 6.8%.
This means that out of the entire incident solar energy bombarding a cell’s surface, only 6.8% can be stored in the form of hydrogen fuel, while the rest will be lost.
On the other hand, the HPEV cells can harvest the residual electrons that do not play a role in fuel generation. It is these leftover electrons which are used for producing electrical power, leading to a remarkable increase in the total solar energy conversion efficiency, Segev said.
For instance, based on the same calculations, it is possible to store the same 6.8% of the solar energy as hydrogen fuel in an HPEV cell made of silicon and bismuth vanadate, and in addition, another 13.4% of the solar energy can also be transformed into electricity.
This allows a total efficiency of 20.2%, which is three times better than traditional solar hydrogen cells.
The researchers intend to pursue their partnership so that they can explore the means of using the HPEV concept for other types of applications, for example, reducing the emissions of carbon dioxide.