Dec 21 2018
Stanford University researchers have created an electrocatalytic mechanism that functions similar to a mammalian lung to turn water into fuel. Published on December 20th, 2018, in the journal Joule, the study could help the current generation of clean energy technologies to operate in a more efficient manner.
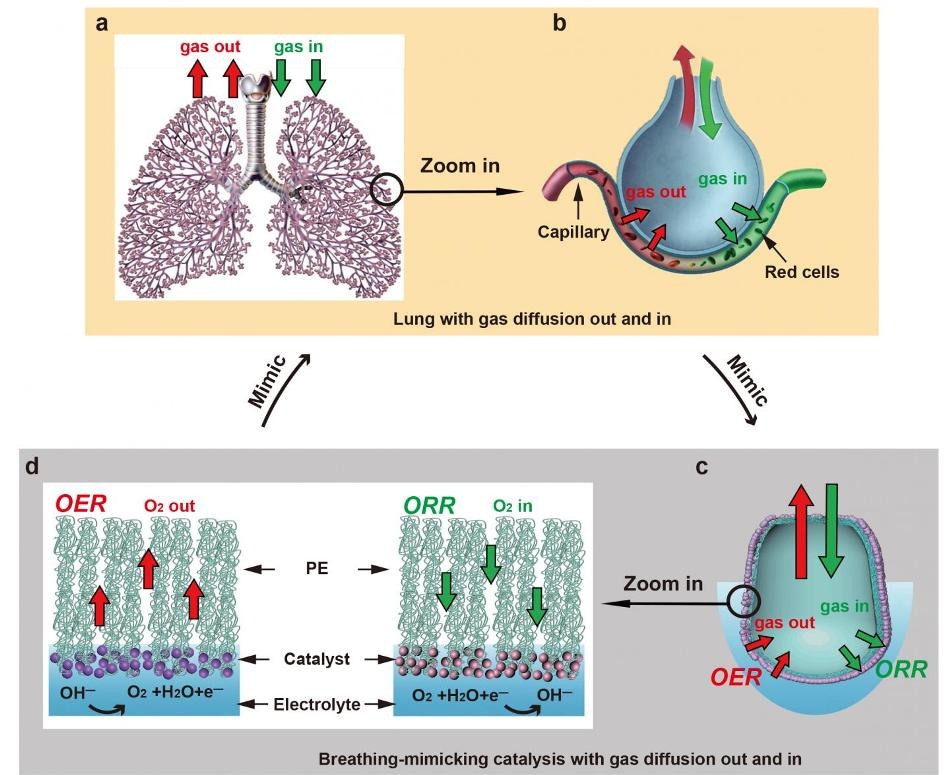 This image shows the similarities between the exchange of gases in mammalian lungs and a newly developed mechanism to turn water into fuel. (Image credit: Li et al./Joule)
This image shows the similarities between the exchange of gases in mammalian lungs and a newly developed mechanism to turn water into fuel. (Image credit: Li et al./Joule)
For a majority of the organisms, exhaling and inhaling is so automatic that it can be mistaken as a simple process; however, the mammalian breathing process is, in fact, one among the most advanced systems for two-way gas exchange that exists in nature. With each breath, air travels through the lungs’ small, passage-like bronchioles until it reaches the tiny sacs known as alveoli. From there, the gas should not simply diffuse but pass into the bloodstream, or else, this would lead to the formation of harmful bubbles.
The special structure of the alveoli—including a micron-thick membrane that has the ability to repel water molecules on the inside and at the same time attracts them on the external surface—not only prevents the formation of those bubbles but also makes the gas exchange extremely efficient.
Inspired from this process, researchers in senior author Yi Cui’s lab at the Department of Materials Science and Engineering at Stanford University successfully developed improved electrocatalysts—materials that increase the speed of a chemical reaction at an electrode.
Clean energy technologies have demonstrated the capability of fast gas reactant delivery to the reaction interface, but the reverse pathway—efficient gas product evolution from the catalyst/electrolyte interface—remains challenging.
Jun Li, Study First Author, Stanford University
The researchers’ mechanism structurally imitates the alveolus and performs two entirely different processes to enhance the reactions that power sustainable technologies like metal-air batteries and fuel cells.
The first process is similar to exhalation. By oxidizing water molecules in a battery’s anode and while reducing them in the cathode, the mechanism splits water to create hydrogen gas—a clean fuel. Oxygen gas, together with the hydrogen gas, is quickly generated and conveyed via a thin, alveolus-like membrane fabricated from polyethylene—but without the energy costs entailed in the formation of bubbles.
The second process is similar to inhalation and produces energy via a reaction that takes up oxygen. Furthermore, oxygen gas is supplied to the catalyst at the surface of the electrode, so that it can be utilized as a reactant at the time of electrochemical reactions.
Even though the design is still in the initial stages of development, it could have potential applications. Compared to traditional carbon-based gas diffusion layers, the unusually thin nano-polyethylene membrane stays hydrophobic for a much longer time. This model has the ability to realize higher rates of current density and lower overpotential when compared to traditional designs.
Yet, more research needs to be done to improve this unique lung-inspired design before it can be applied for commercial applications. The nano-polyethylene membrane cannot withstand temperatures higher than 100 °C since it is a film based on polymers; such temperatures can limit its applications.
According to the researchers, this material can possibly be substituted with analogously thin nanoporous hydrophobic membranes that have the ability to tolerate more extreme heat. The team is also interested in integrating other electrocatalysts within the device design to completely study their catalytic capabilities.
The breathing-mimicking structure could be coupled with many other state-of-the-art electrocatalysts, and further exploration of the gas-liquid-solid three-phase electrode offers exciting opportunities for catalysis.
Jun Li, Study First Author, Stanford University
The Department of Energy, Office of Basic Energy Sciences, Materials Sciences and Engineering Division; the Stanford Nano Shared Facilities; and Stanford Nanofabrication Facility supported the study.