Jan 14 2019
Nearly a century ago a compound called ammonia was first synthesized. Today, it has dozens of uses and has become vital in making the fertilizer that currently sustains most of the worldwide food production.
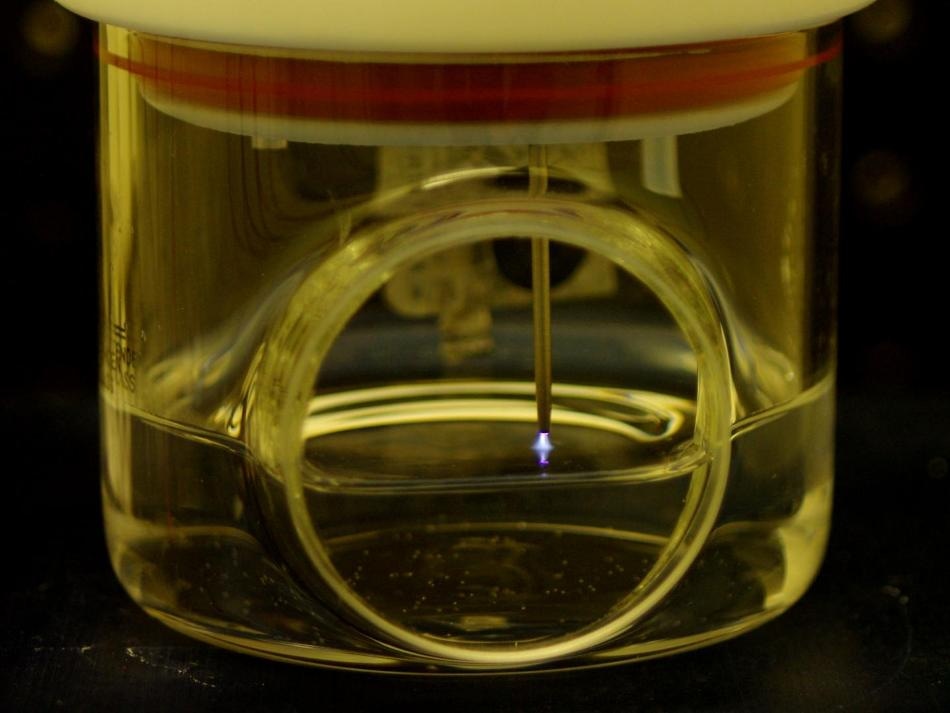 This is a graphic illustration showing protonated water reacting with nitrogen molecules to form ammonia at a plasma-liquid interface. (Image credit: Case Western Reserve University)
This is a graphic illustration showing protonated water reacting with nitrogen molecules to form ammonia at a plasma-liquid interface. (Image credit: Case Western Reserve University)
But while ammonia has been produced at a large scale since the 1930s, it has been accomplished primarily in hulking chemical plants requiring huge amounts of hydrogen gas from fossil fuels—making ammonia among the most energy-intensive of all large-volume chemicals.
Two scientists at Case Western Reserve University—one an expert in electro-chemical synthesis, the other in applications of plasmas—are working on sorting that out.
Scientists Julie Renner and Mohan Sankaran have formulated a new way to produce ammonia from nitrogen and water at low pressure and low temperature. They have done it successfully thus far in a laboratory without using hydrogen or the solid metal catalyst essential in traditional processes.
Our approach—an electrolytic process with a plasma—is completely new.
Mohan Sankaran, Goodrich Professor of Engineering Innovation, Case School of Engineering
Plasmas, frequently referred to as the fourth state of matter (besides solid, liquid, or gas), are ionized clouds of gas, consisting of free electrons and positive ions, which give it the exclusive ability to trigger chemical bonds, including the quite challenging nitrogen molecule, at room temperature.
Renner, a Climo Assistant Professor in the Chemical and Biomolecular Engineering Department, added that because this new method does not need high temperature or high pressure or hydrogen, it makes it scalable—“the ideal kind of technology for a much smaller plant, one with high potential to be powered by renewable energy.”
The results of their two-year partnership were reported recently in the journal Science Advances.
History lesson: The Haber-Bosch process
Almost all commercial ammonia is produced from nitrogen and hydrogen, using an iron catalyst at high temperature and pressure.
German physical chemist Fritz Haber received the Nobel Prize for Chemistry in 1918 for designing this process, which rendered manufacturing ammonia economically practical.
But the process became more cost-effective when industrial chemist Carl Bosch (who also won a Nobel Prize in 1931) brought the technique into a large-scale system. The process was further pushed by a second innovation: the progress of steam methane reforming that made hydrogen more accessible and less costly.
Therefore, what became known as the Haber-Bosch process became the go-to universal technique for fixing nitrogen and hydrogen to form ammonia.
But Haber-Bosch was never the only technique to nitrogen fixation; it was only the turn-of-the-century winner.
A new, old method rises
Renner and Sankaran have revived an element from a virtually unknown Norwegian technique that predated Haber-Bosch (the Birkeland-Eyde process) which reacted nitrogen and oxygen to form nitrates, another chemical that can be employed in agriculture. That process lost out to Haber-Bosch primarily because it needed even more energy in the form of electricity, a limited resource in the early 20th century.
Our approach is similar to electrolytic synthesis of ammonia, which has gained interest as an alternative to Haber-Bosch because it can be integrated with renewable energy. However, like the Birkeland-Eyde process, we use a plasma, which is energy intensive. Electricity is still a barrier, but less so now, and with the increase in renewables, it may not be a barrier at all in the future. And perhaps most significantly, our process does not produce hydrogen gas. This has been the major bottleneck of other electrolytic approaches to forming ammonia from water (and nitrogen), the undesirable formation of hydrogen.
Mohan Sankaran, Goodrich Professor of Engineering Innovation, Case School of Engineering
The Renner-Sankaran process also does not employ a solid metal catalyst that could be one of the reasons ammonia is attained instead of hydrogen.
“In our system, the ammonia is formed at the interface of a gas plasma and liquid water surface and forms freely in solution,” Sankaran said.
Thus far, the “table-top batches” of ammonia produced by the duo have been very tiny and the energy efficiency is still lower than Haber-Bosch. But with continued improvement, their discovery and development of a new process could soon pave the way for smaller, more localized ammonia plants which use green energy.