Mar 15 2019
Renewable sources have to be used efficiently in order to fulfill the growing demand for feedstock chemicals and energy in the future. However, this aspect is considered to be one of the biggest global challenges.
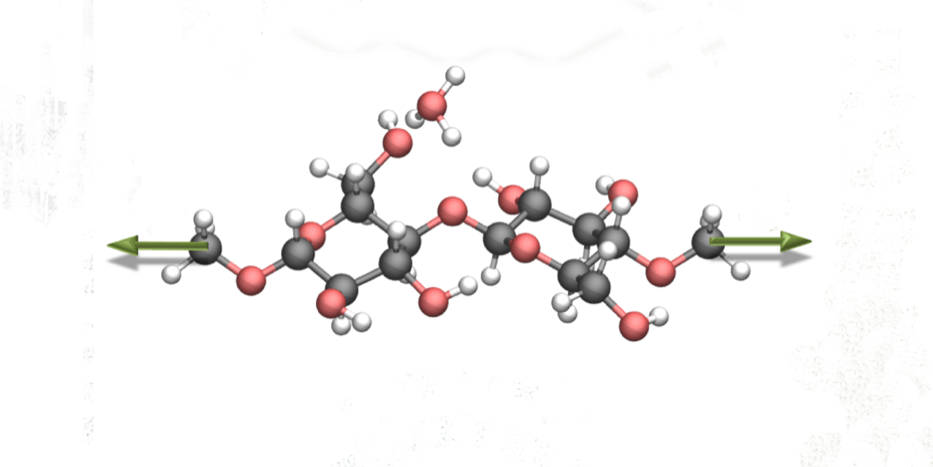 The molecular structure of cellulose, to which nanoscientists applied mechanical force (green arrows). The hydrolysis reaction changed dramatically as a result. (Image credit: Saeed Amirjalayer et al./Angew Chem)
The molecular structure of cellulose, to which nanoscientists applied mechanical force (green arrows). The hydrolysis reaction changed dramatically as a result. (Image credit: Saeed Amirjalayer et al./Angew Chem)
In this regard, biomass offers a potential alternative to current fossil sources like oil or coal. Here, cellulose has an important role to play because it is responsible for the biggest fraction of the storage of natural carbon. Reservoirs like these are important for producing basic chemicals as well as fuels. Therefore, cellulose’s chain-like structure should be broken down to tap its full potential. To acheive this, a so-called hydrolysis reaction can be used, but then this process is rather complicated because of the cellulose’s atomic structure, and added to this, it has been extremely expensive to date.
At the University of Münster, a research team, led by Dr Saeed Amirjalayer and Professor Harald Fuchs and the University of Bochum led by Professor Dominik Marx, has been able to identify a novel reaction mechanism through which cellulose can be efficiently converted by means of mechanical force. Using this so-called mechano-catalytic reaction, a cost-effective, efficient, and environmentally friendly process can possibly be developed for the conversion of biomass.
Backed by the German Research Foundation and the Cluster of Excellence RESOLV at Bochum University, the study has been reported in the journal, Angewandte Chemie International Edition.
Background information and Method:
The backbone of the cellulose can be broken down into separate molecular building block through a hydrolysis reaction. Such molecular building blocks are the real basis for the production of chemical feedstocks or fuels. Researchers, in their quest for identifying ways to render the hydrolysis reaction more efficient, have already discovered proof in previous studies that the conversion processes can be influenced by mechanical forces.
Describing the effect of mechanical force during each separate reaction step at the atomic level has been impossible so far. Yet, this depth of understanding is essential to create a matching efficient process, which is also resource efficient. In the recently published work, the researchers have demonstrated that exerting a mechanical force on the molecules of the cellulose, over some level, has a considerable impact on the reaction.
In order to do that, the researchers performed the so-called atomistic modeling, which allowed them to comprehensively track the individual stages of the hydrolysis reaction, while simultaneously exerting a mechanical force on the molecular structure. The so-called energy profiles detail the energy pathway along the reaction coordinate with and without the effect of mechanical forces. These energy profiles were calculated by the nanoscientists, who eventually succeeded in demonstrating that when the molecular backbone of the cellulose is stressed, it had a major impact on the hydrolysis reaction. While the energy needed to trigger the process was considerably decreased, an increased mechanical force made two of the typical three reaction steps redundant.
By means of our atomistic models we could explicitly investigate the influence of mechanical force on the reaction mechanism. This enabled us to elucidate a previously unknown and highly efficient reaction pathway for the conversion of cellulose.
Dr Saeed Amirjalayer, Study Author and Group Leader, Institute of Applied Physics, Münster University and Center for Nanotechnology.
The latest outcomes prove the experimental observations and also demonstrate the possibility to regulate molecular processes with the aid of mechanical force.
“Among other things, we were able to show that the so-called proton affinity in cellulose can be increased region-selectively by mechanical force,” explained Dr Saeed Amirjalayer.
Thus, according to the researchers, this work will not only promote an environmentally friendly and efficient process for cellulose conversion, but it would also result in the development of new mechano-responsive substances, like plastics. Such substances could be simply recycled through mechanical forces after their usage.