Mar 15 2019
Scientists from China have suggested a method to decrease Uranium concentration in polluted water in a paper to be published in the upcoming issue in NANO. As a vital nuclear fuel, Uranium has been significantly used and unavoidably released to the environment. If not properly disposed of, exposure to uranium can cause grievous harm to human health and the environment.
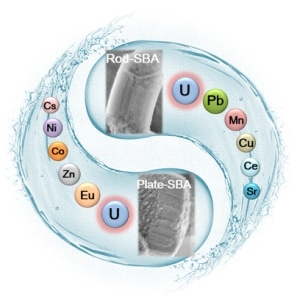 A schematic illustration of the efficient sorption of uranium from aqueous solutions by mesoporous silica SBA-15 with various morphologies. The mesostructures including morphologies and pore length of SBA-15 perform the dominant function for the fast sorption kinetics, while the modified amidoxime groups make the excellent U(VI) sorption capacities and high selectivity possible. (Image credit: World Scientific)
A schematic illustration of the efficient sorption of uranium from aqueous solutions by mesoporous silica SBA-15 with various morphologies. The mesostructures including morphologies and pore length of SBA-15 perform the dominant function for the fast sorption kinetics, while the modified amidoxime groups make the excellent U(VI) sorption capacities and high selectivity possible. (Image credit: World Scientific)
Mesoporous SBA-15 with ordered mesostructures, large pore sizes, and high surface areas have been added to concentrate U(VI) from aqueous solutions. The exploration of SBA-15 with higher performances also keeps progressing. For example, the synthesis of functional SBA-15 with organic ligands comprising N, O, S, and P elements, and with controllable morphologies (for example, plates, rods, and fibers) and tunable mesostructures have also been described. However, the complete evaluation of structure-composition-function relationships, including the interconnected impact of textural features of sorbents and the interaction mechanism of U(VI)-surface altered chemical groups, has not been completely researched yet.
This paper examines the way synergistic incorporation of pore mesochannels and surface functionalization of SBA-15 allows high-performance U(VI) sorption. In this research, the team reports the fast sorption of U(VI) with high capacities and selectivity by amidoxime functionalized ordered mesoporous SBA-15 with two distinctive morphologies (i.e., rods and plates) via a post-grafting technique. The outcomes reveal that the mesostructures including morphologies and pore length of SBA-15 carry out the dominant operation for the rapid sorption kinetics (10 minutes for plates, 20 minutes for rods), while the altered amidoxime groups make superior U(VI) sorption capacities (646.2 mg/g for plates, 499.8 mg/g for rods at pH 5.0, and T 298.15 K) and high selectivity achievable. U(VI) adsorbed amidoxime-functionalized SBA-15 can also be successfully regenerated by HCl solutions and reused extensively even after six cycles, displaying promising potentials for the uptake of radionuclides in real ecological management.
This research was supported by CAS Pioneer Hundred Talents Program, the CASHIPS Director's Fund (No. YZJJ2018QN20), the National Natural Science Foundation of China (Nos. 21875257 and 51708215) and Henan Science and Technology Project (No. 152102310343).
Other co-authors of this research in the journal NANO are Ziyan Yang and Xiaoli Yang form North China University of Water Resources and Electric Power, Junfeng Wu from Henan University of Urban Construction.