Jun 17 2019
An international partnership headed by researchers from Tokyo University of Agriculture and Technology (TUAT), Japan, has created a two-step technique to disintegrate carbohydrates more efficiently into their single sugar components, a crucial process in generating green fuel.
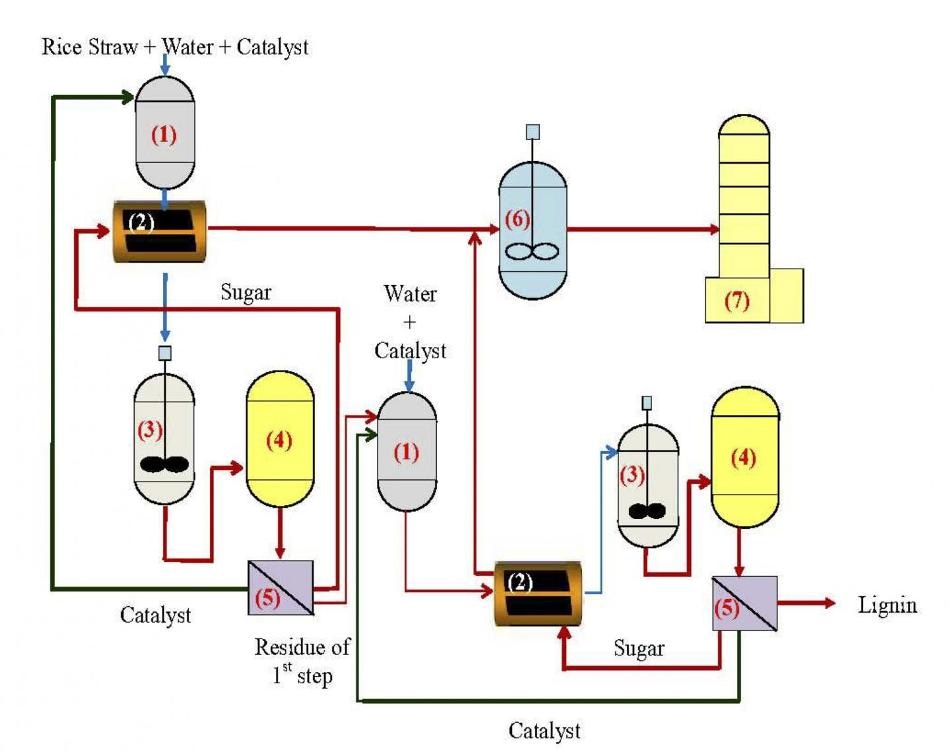 Researchers designed a two-step process to break down rice straws into sugars for fuel. (Image credit: Figure adapted from Ind. Eng. Chem. Res. 2019 58 (14), 5686-5697. Copyright © 2019 American Chemical Society)
Researchers designed a two-step process to break down rice straws into sugars for fuel. (Image credit: Figure adapted from Ind. Eng. Chem. Res. 2019 58 (14), 5686-5697. Copyright © 2019 American Chemical Society)
The scientists reported the study outcomes on April 10th 2019 in the American Chemical Society journal, Industrial & Engineering Chemical Research.
The decomposition process is known as saccharification. The yielded single sugar components known as monosaccharides are fermented into biobutanol or bioethanol, alcohols that can be employed as a fuel.
For a long time, considerable attention has been focused on the utilization of homogenous acids and enzymes for saccharification. Enzymatic saccharification is seen to be a reasonable prospect since it offers the potential for higher yields, lower energy costs, and it's more environmentally friendly.
Eika W. Qian, Study Author and Professor, Graduate School of Bio-Applications and Systems Engineering, Tokyo University of Agriculture and Technology
The breakdown of carbohydrates using enzymes could actually be obstructed, particularly in the practical biomass. For example, rice straw, which is a by-product of rice harvest, comprises of three complicated carbohydrates: hemicelluloses, starch, and cellulose.
Enzymes cannot make contact with hemicellulose or cellulose because of their cell wall structure and surface area, among other characteristics. In order to become receptive to the enzymatic activity, they should be pre-treated, which can be costly.
The use of solid acid catalysts is one solution to overcome the cost and inefficiency of enzymes. These acids bring about chemical reactions without dissolving and becoming a permanent part of the reaction. They are mainly attractive due to the fact that they can be recovered and reused following saccharification.
According to Qian, it is still not as easy as changing the enzymes for the acids, since the carbohydrates are non-uniform. Starch and hemicellulose break down at 180 ○C and below. If the resultant components are further heated, the synthesized sugars degrade and are converted into other byproducts. In contrast, breakdown of cellulose only takes place at temperatures of 200 ○C or more.
For this reason, the scientists devised a two-step process to maximize the resulting yield of sugar from rice straw—one step for hemicellulose and another for cellulose. In the first step, a gentle solid acid is required at low temperatures (150 ○C or less), while in the second step, more adverse conditions are involved, including a stronger solid acid and higher temperatures (210 ○C or more).
On the whole, the two-step process did not just prove effective, it also yielded around 30% more sugars when compared to conventional one-step processes.
“We are now looking for a partner to evaluate the feasibility of our two-step saccharification process in rice straw and other various materials such as wheat straw and corn stoke etc. in a pilot unit,” stated Qian. “Our ultimate goal is to commercialize our process to manufacture monosaccharides from this type of material in the future.”