Aug 1 2019
Recently, the bioconversion of natural gas into liquid fuel has gained increasing interest as a favorable approach. However, the selective hydroxylation of methane—the key component of natural gas—has been one of the crucial challenges for the research community.
 Engineering an enzyme that would uniformly speed along the small alkanes reaction to hydroxyl groups needed to produce fuel. (Image credit: Cong Zhiqi)
Engineering an enzyme that would uniformly speed along the small alkanes reaction to hydroxyl groups needed to produce fuel. (Image credit: Cong Zhiqi)
The development of a biochemical approach with the ability to control the conversion of natural gas into potable liquid fuel by scientists at the Qingdao Institute of Bioenergy and Bioprocesses Technology (QIBEBT) of the Chinese Academy of Sciences has led to the advancement of more sustainable and economic fuel generation. The work has been reported in ACS Catalysis.
Propane (another component of natural gas) and methane are organic molecules known as alkanes. As alkanes comprise only carbon and hydrogen atoms, they need to be carefully processed before using them in fuel. The process involves the addition of hydrogen and oxygen, known as hydroxyl groups, into the alkane. These atoms rearrange themselves and generate an alcohol that could be used as fuel.
The process is indirect because it depends on how selective alkanes are during the reaction with the hydroxyl catalysts. Scientists have synthesized an enzyme that could evenly accelerate the reaction of small alkanes with hydroxyl groups required to generate fuel.
This has been an enduring problem as it is very difficult to directly hydroxylate small alkanes. With existing processing steps, some alkanes are more reactive and make the resulting fuel useless.
To set limits on which alkanes react and to what extent, Prof. Cong Zhiqi from the QIBEBT and his colleagues concentrated on various protein variants of P450 monooxygenase that could potentially facilitate the addition of hydroxyl groups into alkane molecules. Over 41,000 variants of the enzyme could lead to different reaction levels.
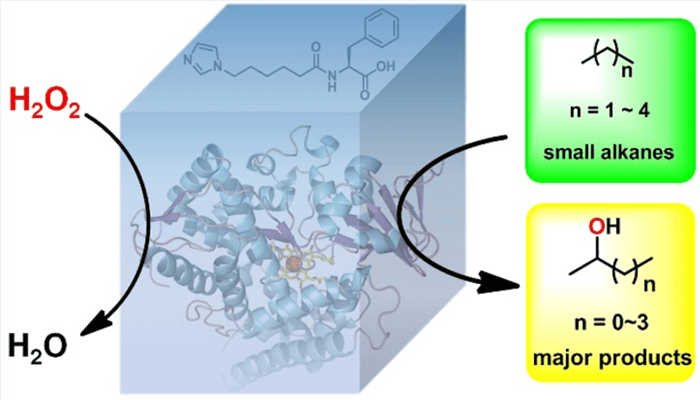
They successfully achieved controllable selective propane hydroxylation through what Prof. Cong describes as a hydrogen peroxide-driven artificial P450 system. This system contains a dual-function small molecule (DFSM), variants of a designed P450 enzyme known as P450BM3, and hydrogen peroxide.
The engineered P45BM3 can react with the hydrogen peroxide, and the DFSM can bind the enzyme and hydrogen peroxide, thereby facilitating the reaction.
The reaction proceeds with the propane and successfully converts the alkanes into alcohols that can be converted into fuel in turn. The scientists discovered that the system had equivalent or better catalytic properties compared to the only known peroxide-dependent natural enzyme of small alkanes, based on which variant of P450BM3 was used.
They produced variants by replacing the substrates on the part of the enzyme that binds to the hydrogen peroxide with more reactive versions, which helped carbon bonds that are otherwise inert to disintegrate and bind with other available atoms.
This study gave the first example of direct small alkane hydroxylation by the peroxide-driven P450BM3 variants. This substantially expands the synthetic toolbox toward the development of a practical catalyst for fuel processing. We hope we can further tune the enzyme for use in methane oxidation, as well.
Cong Zhiqi, Professor, Qingdao Institute of Bioenergy and Bioprocesses Technology (QIBEBT), Chinese Academy of Sciences
The scientists are currently investigating the particular molecular reaction mechanisms and planning to use the data to design similar systems for use with other natural gas components like methane.