Aug 5 2019
Engineers at Lehigh University are the first to apply a single enzyme biomineralization process to develop a catalyst that uses the energy of trapped sunlight to divide water molecules to create hydrogen. The synthesis process is carried out at room temperature and under ambient pressure, solving the scalability and sustainability challenges of formerly reported techniques.
 (Image credit: Green Chemistry)
(Image credit: Green Chemistry)
Solar-driven water splitting is a favorable route towards an economy based on renewable energy. The generated hydrogen could serve as a transportation fuel as well as a critical chemical feedstock for chemical and fertilizer production. Both of these sectors currently add to a large fraction of total greenhouse gas emissions.
One of the difficulties to attaining the promise of solar-driven energy production is that, while the necessary water is a rich resource, formerly explored approaches utilize complicated routes that necessitate environmentally damaging solvents and huge amounts of energy to create at large scale. The cost and harm to the environment have rendered these techniques ineffectual as a long-term solution.
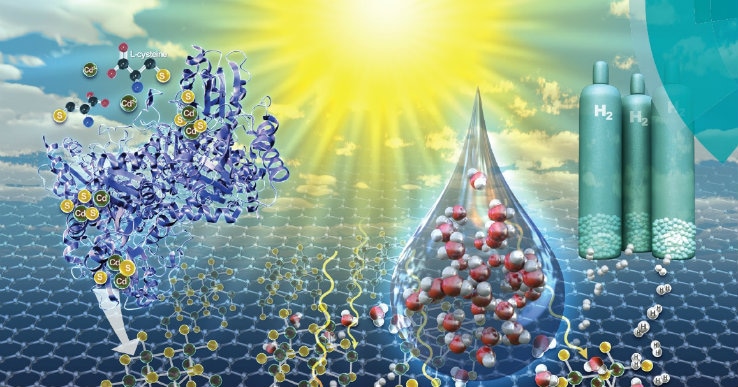
A team of engineers at Lehigh University have harnessed a biomineralization method to manufacturing both quantum-confined nanoparticle metal sulfide particles and the supporting reduced graphene oxide material to make a photocatalyst that splits water to form hydrogen. The team described their results in an article entitled: “Enzymatic synthesis of supported CdS quantum dot/reduced graphene oxide photocatalysts” featured on the cover of Green Chemistry, a journal of the Royal Society of Chemistry on August 7th.
The authors of the research paper include Steven McIntosh, Professor in Lehigh’s Department of Chemical and Biomolecular Engineering, along with Leah C. Spangler, former Ph.D. student and John D. Sakizadeh, current Ph.D. student; as well, as Christopher J. Kiely, Harold B. Chambers Senior Professor in Lehigh’s Department of Materials Science and Engineering and Joseph P. Cline, a Ph.D. student working with Kiely.
“Our water-based process represents a scalable green route for the production of this promising photocatalyst technology,” said McIntosh, who is also Associate Director of Lehigh’s Institute for Functional Materials and Devices.
Some years ago, McIntosh’s team formulated a single enzyme method for biomineralization―the process by which living organisms create minerals―of size-controlled, quantum-confined metal sulfide nanocrystals. In an earlier partnership with Kiely, the lab effectively showed the first precisely regulated, biological way to produce quantum dots.
Their one-step technique started with engineered bacterial cells in a standard, aqueous solution and ended with functional semiconducting nanoparticles, all without utilizing high temperatures and harmful chemicals. The technique was covered in a New York Times article: “How a Mysterious Bacteria Almost Gave You a Better TV.”
Other groups have experimented with biomineralization for chemical synthesis of nanomaterials. The challenge has been achieving control over the properties of the materials such as particle size and crystallinity so that the resulting material can be used in energy applications.
Leah C. Spangler, Lead Author and Postdoctoral Research Fellow, Princeton University
McIntosh explains how Spangler was able to tweak the team’s proven biomineralization process to not only synthesize the cadmium sulfide nanoparticles but also to decrease graphene oxide to the more conductive reduced graphene oxide form.
She was then able to bind the two components together to create a more efficient photocatalyst consisting of the nanoparticles supported on the reduced graphene oxide. Thus her hard work and resulting discovery enabled both critical components for the photocatalyst to be synthesized in a green manner.
Steven McIntosh, Study Author and Professor, Department of Chemical and Biomolecular Engineering, Lehigh University
The team’s research reveals the utility of biomineralization to accomplish the benign synthesis of functional materials for application in the energy sector.
“Industry may consider implementation of such novel synthesis routes at scale,” adds Kiely. “Other scientists may also be able to utilize the concepts in this work to create other materials of critical technological importance.”
McIntosh stresses the potential of this promising new technique as “a green route, to a green energy source, using abundant resources.”
“It is critical to recognize that any practical solution to the greening of our energy sector will have to be implemented at enormous scale to have any substantial impact,” he adds.