Oct 14 2019
NUS chemists have developed carbon-conjugated covalent organic frameworks for visible light-driven catalytic production of hydrogen gas from water.
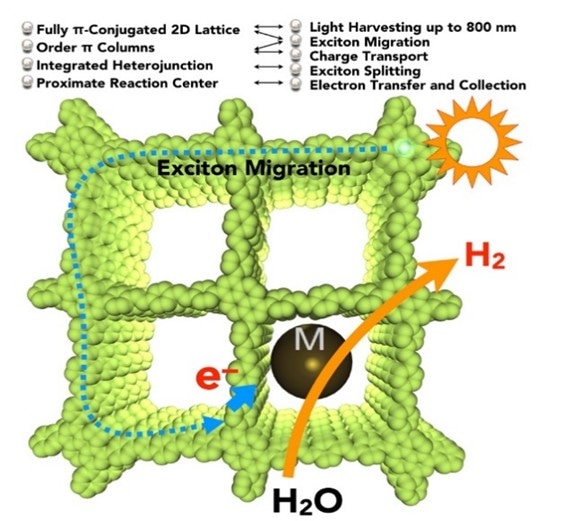 Illustration of the carbon-conjugated covalent organic framework (COF) showing the photocatalytic system in which a wide range of visible light can be harvested for the production of hydrogen gas from water. Nanoparticles (denoted by M) can be loaded into the COF as a reaction centre. [Credit: Chem]
Illustration of the carbon-conjugated covalent organic framework (COF) showing the photocatalytic system in which a wide range of visible light can be harvested for the production of hydrogen gas from water. Nanoparticles (denoted by M) can be loaded into the COF as a reaction centre. [Credit: Chem]
Hydrogen gas is becoming important as a storage medium for sustainable energy applications. The use of sunlight, a renewable and sustainable energy source to decompose water into hydrogen gas is attracting significant scientific interest. However, this conversion from water to hydrogen gas does not happen spontaneously. It requires a complex system that involves a flow of free electrons generated by the light source that acts as an electric current to split the water molecule.
The research team led by Prof JIANG Donglin from the Department of Chemistry, NUS has developed a new class of photocatalysts using carbon-conjugated covalent organic frameworks (COF) for hydrogen gas production from water using solar energy. The research team constructed an organic yet robust material in which carbon-based building blocks are connected by specific bonds in a topologically predesigned ordered manner. This unique molecular structure looks like stacked layers of two-dimensional networks and is able to harvest sunlight efficiently. The researchers inserted platinum nanoparticles as reaction centres into the COF and under visible light irradiation (≥ 420 nm), hydrogen gas was generated at a steady rate of 1,360 μmol h-1g-1 over a period of five hours.
The newly developed photocatalyst has several molecular mechanisms that allow it to produce hydrogen gas from water efficiently. It consist of sp2 carbon frameworks that are π-conjugated with low energy bandgaps. This allows the absorption of light from the visible to the near infrared spectrum. The researchers also engineered the periphery (outermost edge position) of the layered two-dimensional lattice with electron-deficient units to synthetically control the electronic and photoelectric properties of the photocatalyst. Moreover, as the structure has dense and ordered columnar π-arrays, these provide pathways to facilitate excitons (an exciton is a bound state of an electron-hole pair) migration and charge transport.
Prof Jiang said, “Nanoparticles such as platinum can be loaded in the pores or on the surface of the photocatalyst to act as reaction centres. This shortens the electron transfer distance and promotes the accumulation of electrons, improving the conversion performance.”
“We anticipate that this work may offer the structural and mechanistic base for scalable and sustainable fuel production from water and sunlight,” added Prof Jiang.