Dec 6 2019
A group of industrial chemicals referred to as “PFAS” has penetrated the far reaches of the Earth, with implications that researchers are just beginning to comprehend.
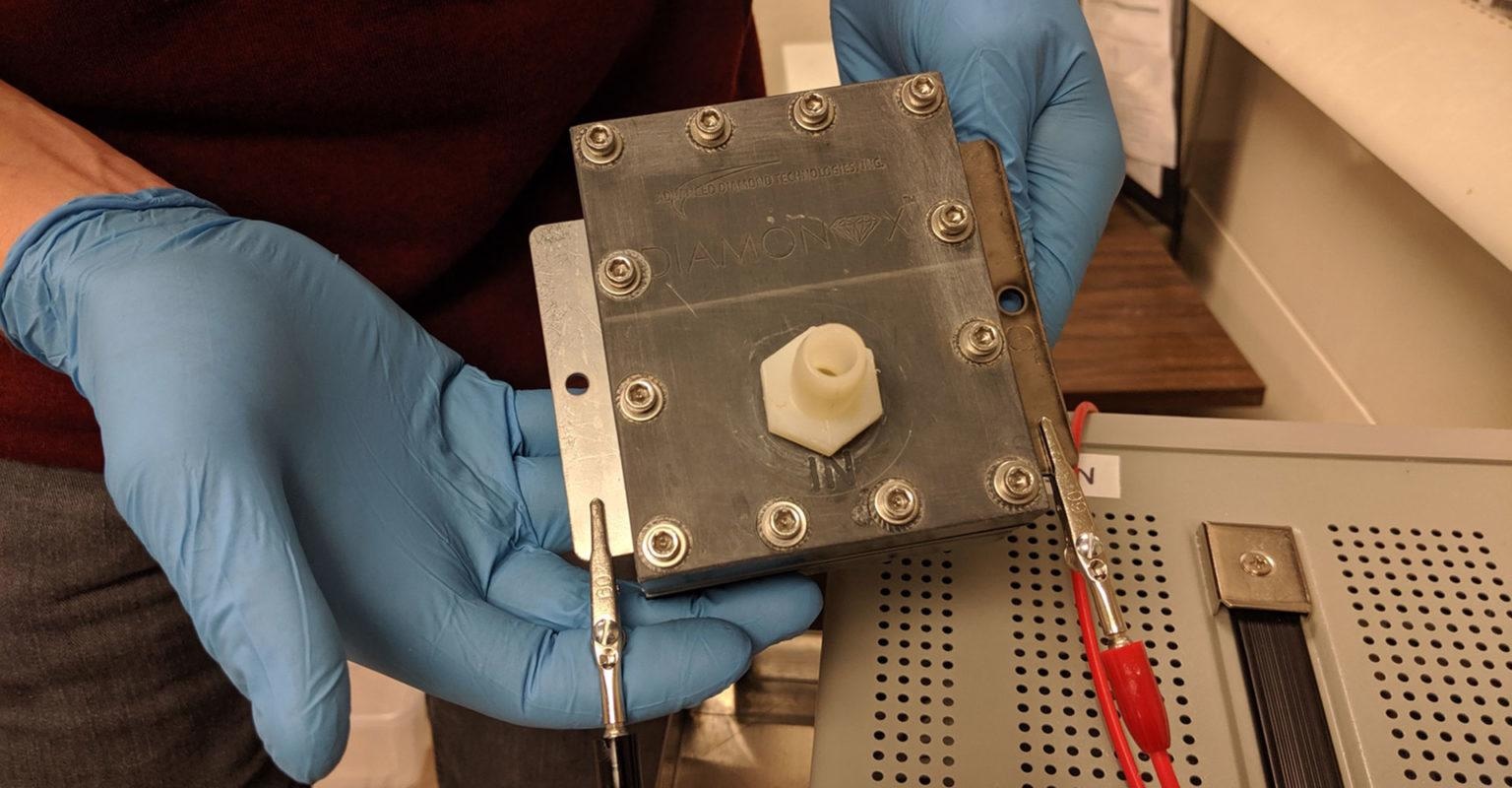 An electrochemical flow cell with a stainless steel cathode and a boron-doped diamond anode is used to treat a concentrated waste stream of GenX. Image Credit: Colorado State University.
An electrochemical flow cell with a stainless steel cathode and a boron-doped diamond anode is used to treat a concentrated waste stream of GenX. Image Credit: Colorado State University.
PFAS—Per- and polyfluoroalkyl substances—are man-made fluorine compounds that have provided consumers with non-stick coatings, waxes, polishes, firefighting foams, and cleaning products used at military bases and airports. They are found in consumer goods like popcorn bags, carpets, water-repellent shoes, and wall paint. They have a vital role in the aerospace, electronics, telecommunications, automotive, data storage, and healthcare industries.
The carbon-fluorine chemical bond, which is one of the strongest bonds in nature, is the reason behind the immense success of these chemicals and the vast environmental challenges they have caused since the 1940s. PFAS residues are present even in some of the most unpolluted water sources, and in the tissue of polar bears.
Industry and science are asked to clean up these steadfast chemicals, a few of which, in definite quantities, have been associated with negative health effects for humans and animals.
Of those striving to solve this extremely tough problem are engineers in the Walter Scott, Jr. College of Engineering at Colorado State University. CSU is one of a very few institutions with the advanced instrumentation and expertise to explore PFAS by revealing their presence in unbelievably trace quantities.
Cleaning up “GenX”
At present, CSU engineers headed by Jens Blotevogel, research assistant professor in the Department of Civil and Environmental Engineering, have reported a new set of experiments dealing with a specific PFAS compound known as hexafluoropropylene oxide dimer acid, better known by its trade name, GenX.
The chemical, and other polymerization processes that use analogous chemistries, have been used for nearly 10 years. They were developed as an alternative for traditional PFAS chemicals called “C8” compounds that were—and still are—mainly persistent in soil and water, and very hard to clean up (thus their nickname, “forever chemicals”).
GenX has become a domestic name in the Cape Fear basin area of North Carolina, where it was found in the local drinking water a few years ago. Chemours, the company responsible for the pollution, has committed to decreasing fluorinated organic chemicals in local air emissions by 99.99%, and air and water emissions from its worldwide operations by at least 99% by 2030.
In the past several years, Chemours has also sponsored Blotevogel’s team at CSU as they test novel approaches that would aid the environment as well as help the company’s legacy cleanup responsibilities.
Treatment Train
Reporting the study in Environmental Science and Technology, Blotevogel collaborated with Tiezheng Tong, assistant professor in civil and environmental engineering, to show an effective “treatment train” that integrates numerous technologies to accurately segregate and destroy GenX residues in water.
One of the existing practices to treat GenX-contaminated water is high-temperature incineration—a procedure that is “excessively expensive,” according to the scientists, and highly wasteful for water and energy recovery. “It works,” Blotevogel said, “but it’s not sustainable.”
The scientists are offering an improved solution. Tong, a pioneering expert in membrane filtration and desalination approaches for environmental risks, utilized a nanofiltration membrane with suitable pore sizes to filter out 99.5% of dissolved GenX compounds.
As soon as the concentrated waste stream is produced, the scientists demonstrated that electrochemical oxidation, which Blotevogel regards to be one of the most feasible technologies for destructive PFAS cleanup, can then decompose the waste into harmless products.
At present, companies can also use many measures for the elimination of PFAS from water to admissible levels: ion exchange, adsorption to activated carbon, and reverse osmosis. While all three of these technologies can be very fruitful, they do not destroy PFAS compounds directly, stated Blotevogel.
The CSU team’s alternative solution of electrochemical treatment employs electrodes to chemically alter the PFAS into more benign compounds. Blotevogel’s lab has shown several positive pilot-scale decontamination efforts, and is working to continue enhancing their methodologies.
Integrated with Tong’s nanofiltration system, the waste stream would be directed and concentrated, which would result in saving companies money and minimizing the carbon footprint of the whole process.
The scientists are keen on continuing to work together to improve their process, for example, by testing various types of filtration membranes to establish the most ideal materials and design.