Feb 13 2020
Petra Rudolf, a Professor of Experimental Solid State Physics at the University of Groningen, has created neatly spaced slits in a clay mineral that allows the filtering of water and removal of harmful herbicide.
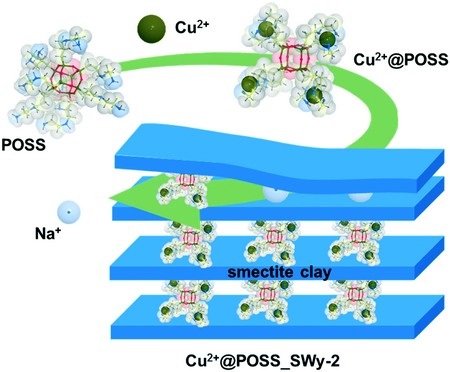 Schematic representation of the production of the modified clay. Image Credit: Feng Yan et al.
Schematic representation of the production of the modified clay. Image Credit: Feng Yan et al.
The clay can be reused once the pollutant is removed by heating the material. Rudolf and collaborators from Greece have described this proof of principle study in the Environmental Science journal.
An unexpected meeting at the Groningen branch of Zonta—an organization of professionals empowering women across the world—led Rudolf to a research question. “At this meeting, I got into a conversation with another member and she asked me about my work,” she stated.
This member was the Director of Waterlaboratorium Noord (Northern Water Laboratory)—Hilde Prummel. When Prummel came to know that part of Rudolf’s study involved the production of materials with pores that are capable of absorbing particular substances, she shared an issue that she faced in water quality control.
A large number of sugar beets are grown in the Northern part of the Netherlands. In these fields, a type of herbicide called chloridazon is extensively utilized. But this compound does not decompose naturally, is harmful to humans, and will ultimately enter the groundwater.
At present, the concentrations of chloridazon in groundwater are below the safety threshold but they are likely to increase because of their persistence in the environment.
Water purification plants can break down chloridazon using UV light—but the breakdown products of chloridazon are also toxic.
Petra Rudolf, Professor of Experimental Solid State Physics, University of Groningen
Rudolf has now developed a method to render well-defined nanocavities in clay, which she modified to trap the herbicide.
“Clay is a layered minera,” explained Rudolf. “The layers have a negative charge and are separated by positive ions. We can replace those with molecular pillars of our own design.”
The natural clays are initially cleaned and subsequently treated with sodium salts. The sodium substitutes the natural positive ions present between the layers.
These sodium ions are surrounded by a water mantle, which pushes the layers slightly further apart. By simply adding the pillar molecules to the water, they will replace the sodium.
Petra Rudolf, Professor of Experimental Solid State Physics, University of Groningen
These molecular pillars are often composed of silicon oxide, with an additional chemical group defining the cavities’ affinity.
Rudolf added that, “In this case, we added copper ions to attract the chloridazon and its breakdown products.” The functionalized clay was able to absorb the herbicide in large amounts: almost 900 mg/kg of clay. “This is a good result and we see scope to further increase the absorption.”
Rudolf and her collaborators have also demonstrated that the clay can be heated to remove the herbicide and the same clay be utilized again.
By altering the affinity of the pillars and changing the thickness of the slits, different chemical compounds can be captured by the functionalized clay.
We are testing systems to remove two other compounds from water. Furthermore, a similar system could be created using other layered materials, such as graphene oxide.
Petra Rudolf, Professor of Experimental Solid State Physics, University of Groningen